NEW! Biorelevant Discriminatory dissolution for Exemestane 25mg tablets
Background
In vitro biorelevant dissolution tests were conducted on the steroidal anticancer molecule exemestane (Reference drug product Aromasin®) and a generic formulation widely marketed in Europe.
The drug products (Reference and Test) from the originator and a generic supplier were sourced from the UK. The pharmacokinetic (PK) parameters of the exact two formulations under fasting conditions in 64 post-menopausal female subjects were obtained from the EU Public Assessment Report for the generic Test product.
The in vivo bioavailability of the two exemestane tablets was translated in the in vivo dissolution findings.

Comparative Dissolution profiles of the Reference and Test product were determined in the fasted state medium FaSSIF using our Biorelevant Dissolution Guide.
Analytical and dissolution method
An HPLC method was successfully developed for selectivity, linearity, precision and accuracy. Samples were found to be stable for the duration of the analysis. The dissolution method was found to be accurate and precise using a USP 2 apparatus. Both sets of dissolution tests used the same parameters (n=6, 75rpm, 900 mL volume and 37°C).
In vitro dissolution
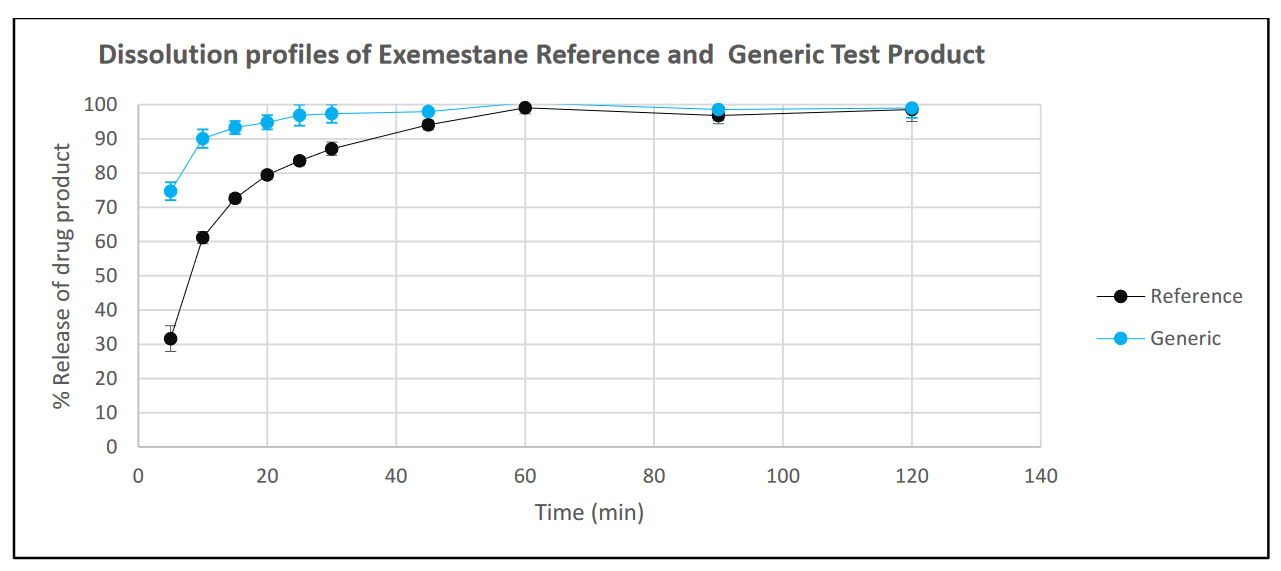
In fasted state simulated intestinal medium, the dissolution release profiles of both Test formulation and Reference are quite fast: within 20 minutes more than 80% of drug is released from both formulations. However, the dissolution of the Test (generic) product is significantly quicker than the Reference. In this example, results are shown only up to 2 hours as the release plateaued after 1 hour.
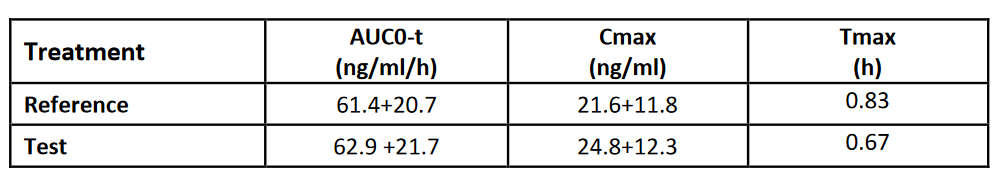
Summary of PK Parameters (non-transformed; arithmetic mean +/- SD))
Although other biological and physiological factors, such as metabolism and gender, influence bioavailability, for this drug the dissolution/release of the drug from the formulations into the intestinal fluid is an important factor affecting absorption and subsequent bioavailabilty. Biorelevant dissolution studies in simulated fluids enable this process to be examined in vitro without other physiological interferences.
AUC, Cmax and Tmax
Results in a appropriately selected biorelevant medium can clearly discriminate a significant in vitro difference between the Test and Reference which can be reflected by the in vivo pharmacokinetics.
Similar in vivo AUC values indicate that in the fasted state the drug in the reference and the test product are available for absorption to a similar extent. This is reflected by the fact that after about an hour the in vitro dissolution reaches completion.
In contrast, the higher in vivo Cmax of the Test is reflected by the faster in vitro dissolution profile compared with the Reference.
The relatively fast in vivo Tmax (<1hr) is reflected by the fast in vitro dissolution of both formulations, the Test Product being slightly faster than the Reference.
Discriminatory power
This in vitro study illustrates the discriminatory power of biorelevant media for the immediate release test drug product exemestane when the correct medium is selected.
















 Home
Home















