
FEDGAS (Early/Mid/Late)
-
Biorelevant dissolution media simulating gastric fluids after food
-
Excellent discriminatory power for neutral, basic or acidic BCS Class 2 drugs
-
Reduce the risk of bioequivalence (BE) failures for water insoluble drugs
-
900mL of FEDGAS contains the 62.5g of fat present in a high-fat FDA meal
-
Different pH’s (6, 4.5 and 3) reflect Early/Mid/Late stages of stomach emptying
-
Designed for use in standard USP Dissolution 1 and 2 apparatus
-
Each bottle of Gel +Buffer Concentrate makes 6L of dissolution medium
-
Smaller FEDGAS Gel bottle size now available
-
Add custom Syringe Filters in packs of 72 pieces










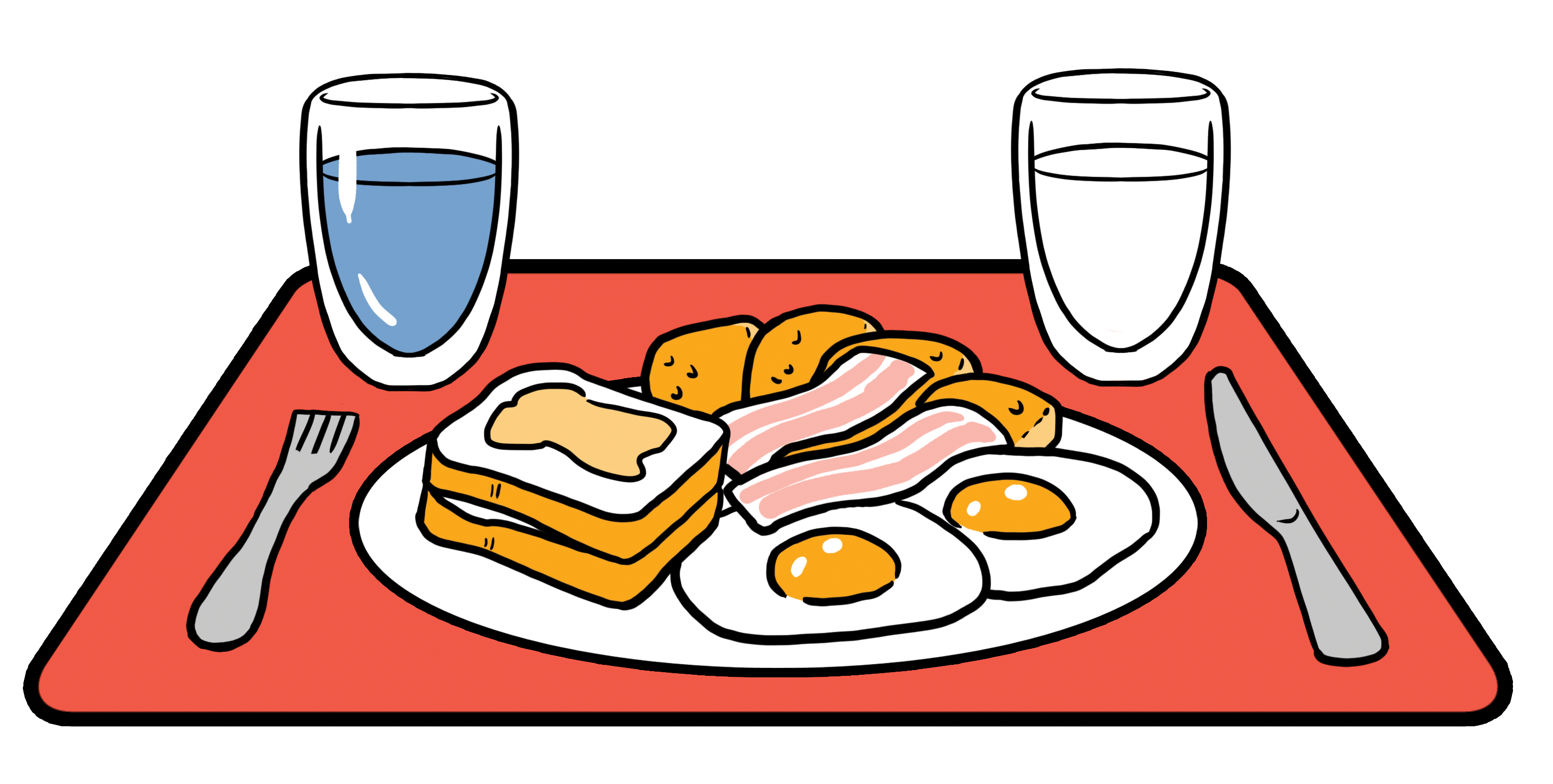
900mL of FEDGAS contains the 62.5g of fat present in a high-fat FDA meal along with the bile salts present in the fed stomach









Use in combination with FEDGAS Buffer Concentrate of your choice




































pH values represent different stages of stomach emptying: Early (6), Mid (4.5) and Late (3)
Use in combination with FEDGAS Gel

High filtration efficiency with minimal risk of clogging or bursting

Low drug adsorption and negligible extractables









High filtration efficiency with minimal risk of clogging or bursting

Low drug adsorption and negligible extractables








For filtration of dissolution samples <5mL we recommend 13mm version
For filtration of dissolution samples >5mL we recommend 25mm version
We have emailed you the results
Error while sending the email
Please try again
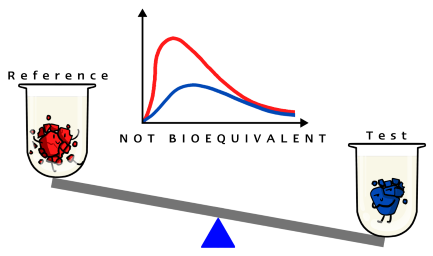
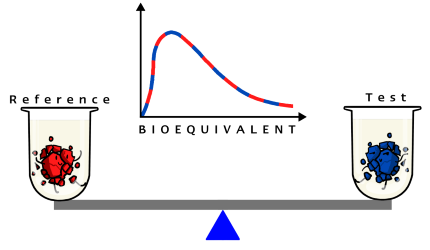
FEDGAS simulates fed state gastric fluids. You can harness its power in your laboratory to discriminate significant formulation differences between your Test Product and the Reference. 900ml of this biorelevant dissolution medium contains the 62.5g of fat present in the high-fat meal along with bile salts present at physiological levels found in the fed stomach. Testing in it is particularly useful for lipophilic water insoluble drugs because good physiological dissolution conditions are created for these drugs.
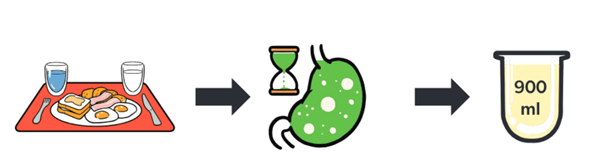
Three types of fed state gastric media (at pH 6, pH 4.5 and pH 3) can easily be prepared using FEDGAS Gel and the appropriate diluted FEDGAS Buffer Concentrate. These three dissolution media reflect the different states of stomach emptying:
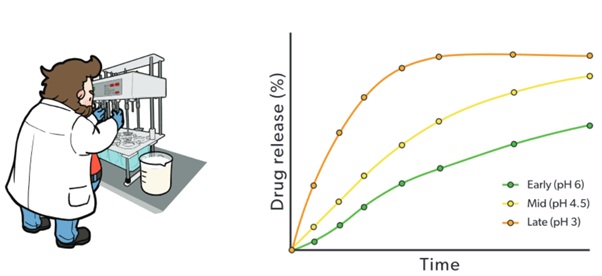
Generating and comparing dissolution profiles in the right biorelevant media helps reduce the risk of an expensive failed human bioequivalence study due to differences in formulation. Matching the dissolution profile of your Test Formulation to the Originator in FEDGAS will maximise the chances of achieving successful bioequivalence. In vitro biorelevant dissolution helps identify a Test formulation that matches the release rate of the Reference in vivo.
You can download dedicated FEDGAS guides for drug analysis and dissolution here.



















 Home
Home















