
FaSSIF/FeSSIF/FaSSGF
-
Use 3F Powder + Buffer Concentrates to make FaSSIF, FeSSIF or FaSSGF
-
3F Powder contains bile salts + lecithin to form gastrointestinal mixed micelles
-
Use Media Preparation Tool below for customized preparation instructions
-
Custom Syringe Filters available in packs of 72 pieces
























70g of 3F Powder makes FaSSIF (30L) or FeSSIF (6L) or FaSSGF (>1000L)
7g of 3F Powder makes FaSSIF (3L) or FeSSIF (600mL) or FaSSGF (>100L)

FaSSIF= Fasted State Simulated Intestinal Fluid










FeSSIF=Fed State Simulated Intestinal Fluid










FaSSGF=Fasted State Simulated Gastric Fluid









Use with 3F Powder to make FaSSIF/FeSSIF/FaSSGF in seconds


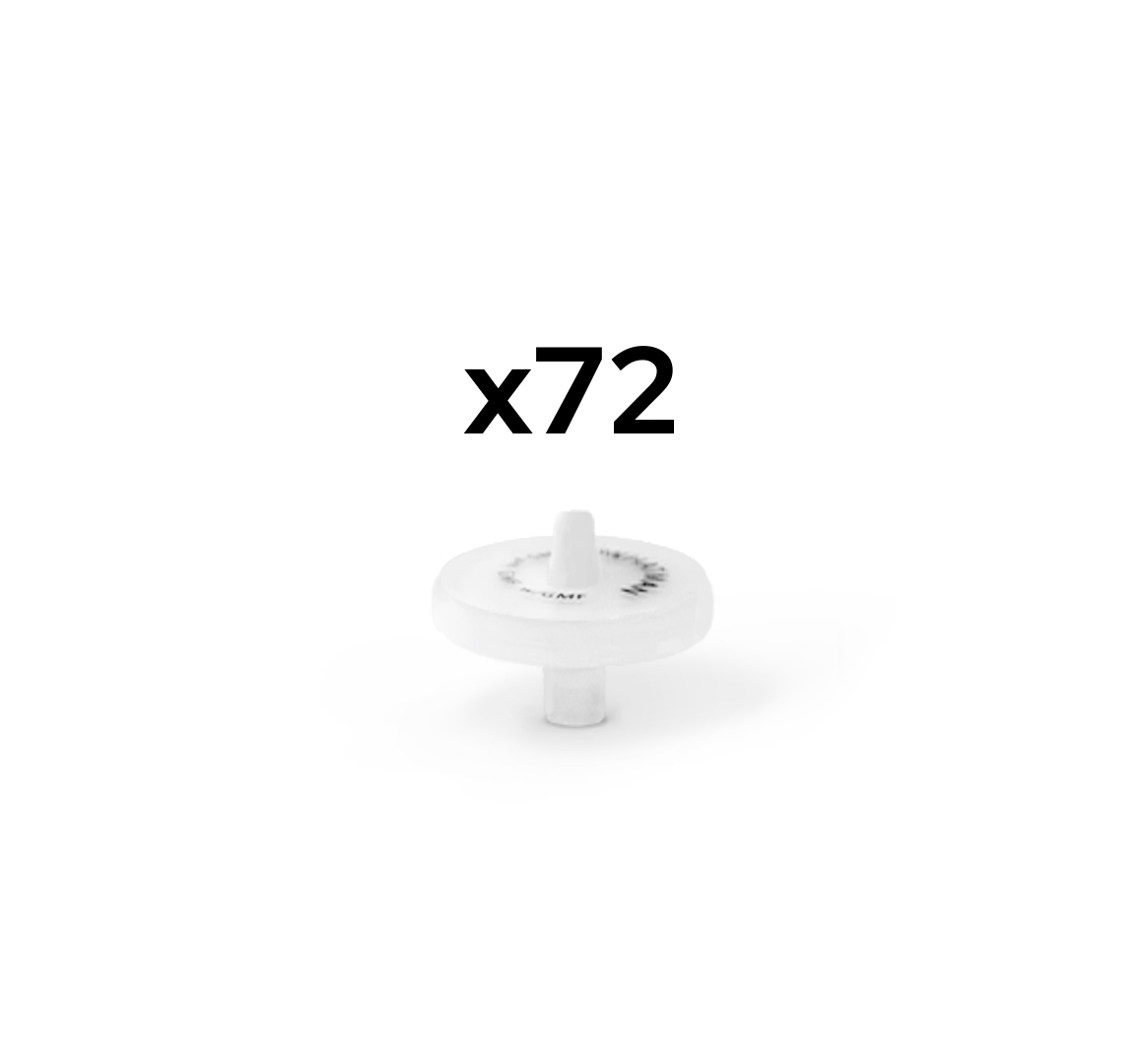








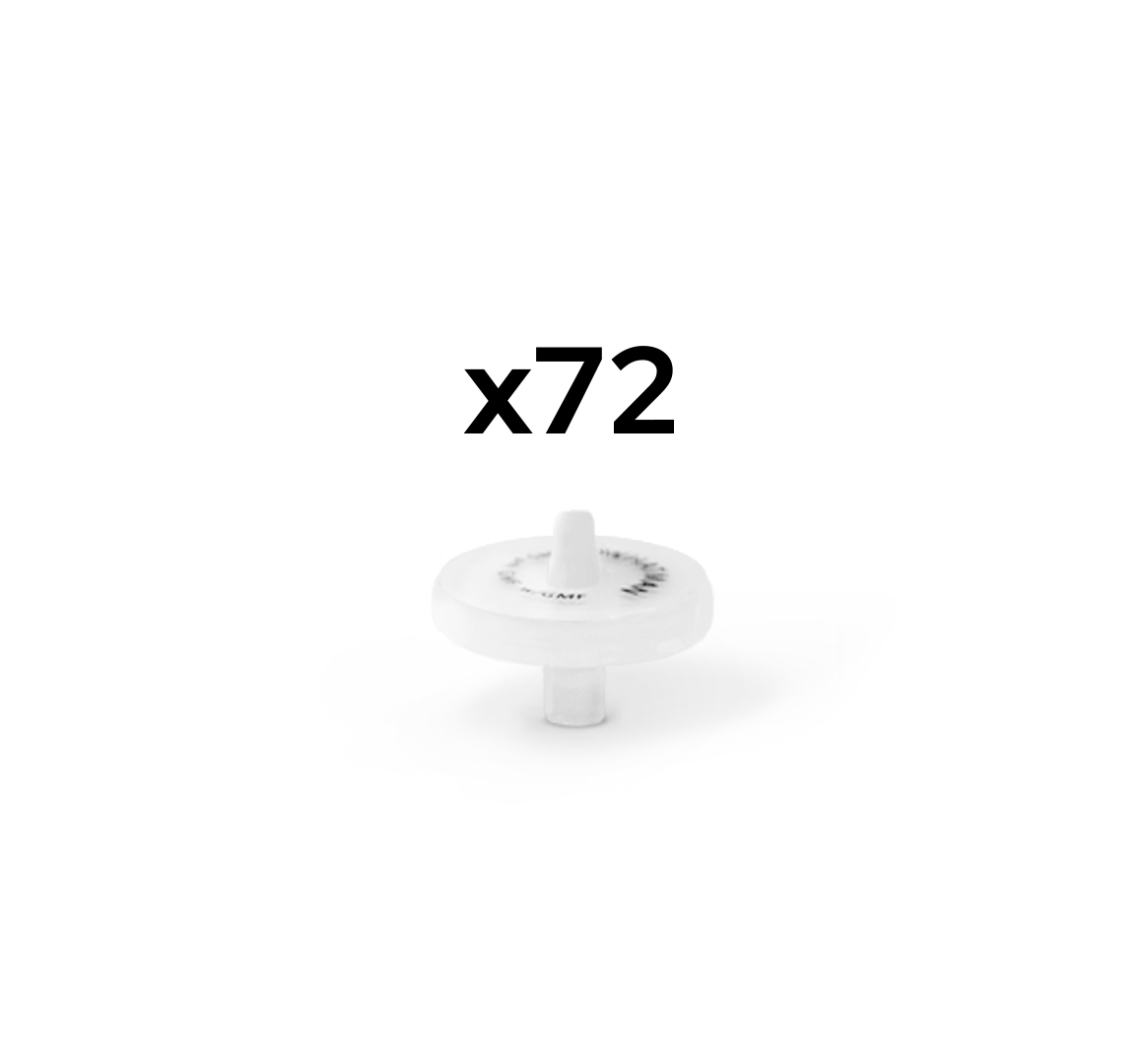

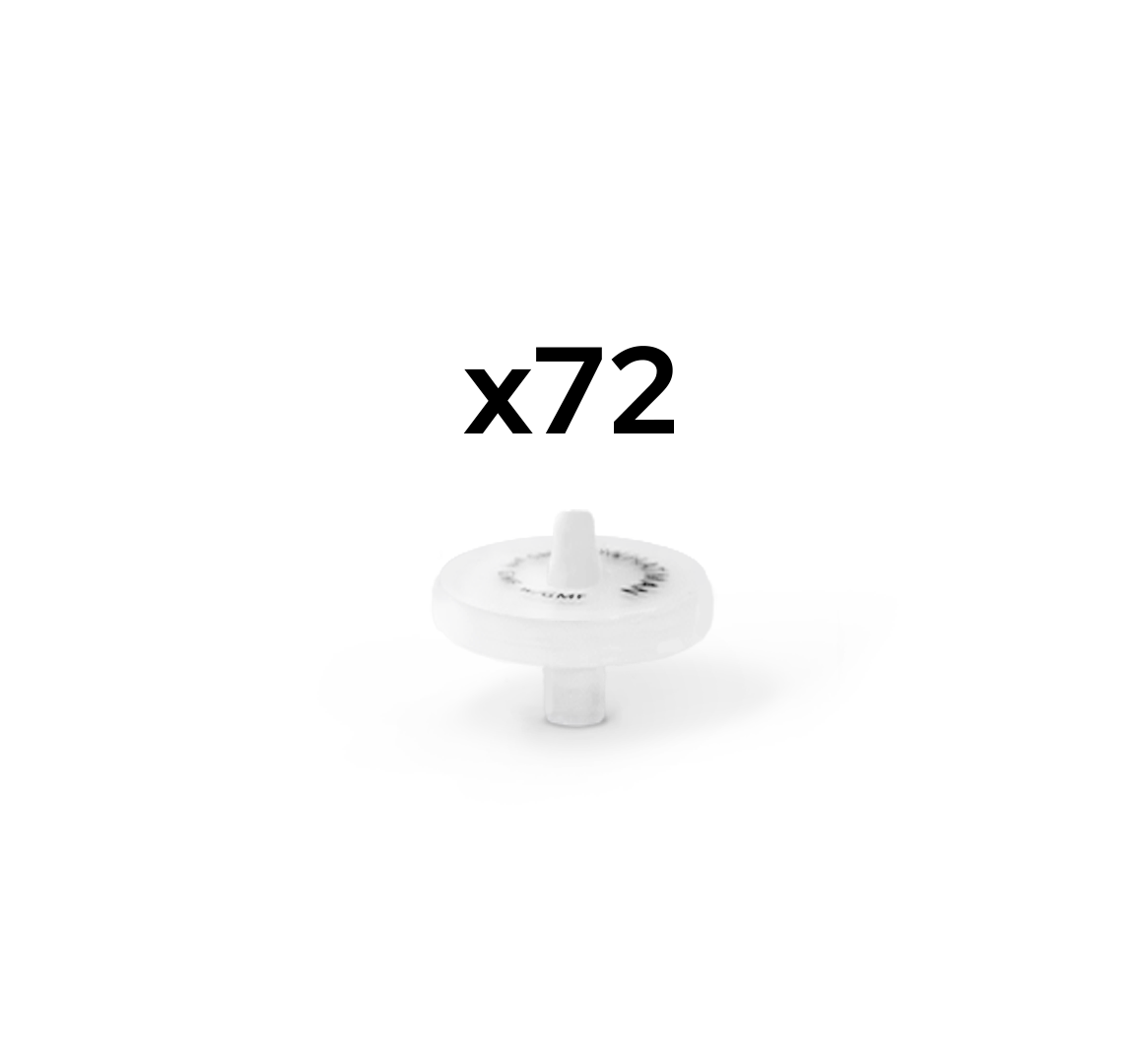







For filtration of dissolution samples <5mL we recommend 13mm version
For filtration of dissolution samples >5mL we recommend 25mm version
We have emailed you the results
Error while sending the email
Please try again
Product Description
FaSSIF, FeSSIF and FaSSGF are dissolution media that simulate human gut fluids:
FaSSIF: Fasted State Simulated Intestinal Fluid
FeSSIF: Fed State Simulated Intestinal Fluid
FaSSGF: Fasted State Simulated Gastric Fluid
They contain physiological surfactants (bile salts and lecithin) present in the gastrointestinal tract to simulate these fluids much more accurately than conventional dissolution media.
We’ve been producing FaSSIF/FeSSIF/FaSSGF (originally known as ‘SIF Powder’) for more than a decade and it’s trusted by the 10 biggest Pharmaceutical companies in the world as the industrial standard for preparing biorelevant test media. Its versatility and ease of use make it the perfect introduction to biorelevant testing. Here’s why the product is so useful to both new and generic developers of oral drugs:
*In vitro solubility results in biorelevant media help establish a relationship between the biopharmaceutical properties of an oral drug and in vivo absorption
*In vitro dissolution results in biorelevant media can enable drug developers to identify test formulations with superior dissolution profiles
*Ideal for use in USP 1 or USP 2 dissolution apparatus (we recommend 900mL of media per vessel)
*Drug analysis can be carried out by HPLC
*Standardized preparation method is extremely fast and simple and results in highly reproducible media every time
*Get customized instructions explaining how to make different FaSSIF/FeSSIF/FaSSGF volumes from our ‘Media Preparation Tool’ (see above)
















 Home
Home















