
Two Stage Dissolution: FaSSGF→FaSSIF
-
Simulate how a drug product behaves when it contacts the stomach fluid (FaSSGF) and then when it is converted into intestinal fluid (FaSSIF)
-
Results will reveal the drug’s tendency to either supersaturate or precipitate
-
Particularly important for basic drugs with a higher solubility at acidic stomach pH compared to higher intestinal pH
-
Will help you make important formulation development decisions to optimize gastrointestinal absorption of poorly water-soluble drugs in the fasted state
Background
For a drug to be absorbed, it has to dissolve in vivo in intestinal fluids. To investigate drug supersaturation/precipitation, we recommend two different dissolution studies:
1) Establish dissolution of the drug product in FaSSIF (Fasted State Simulated Intestinal Fluid) to understand drug dissolution without interference from stomach fluids.
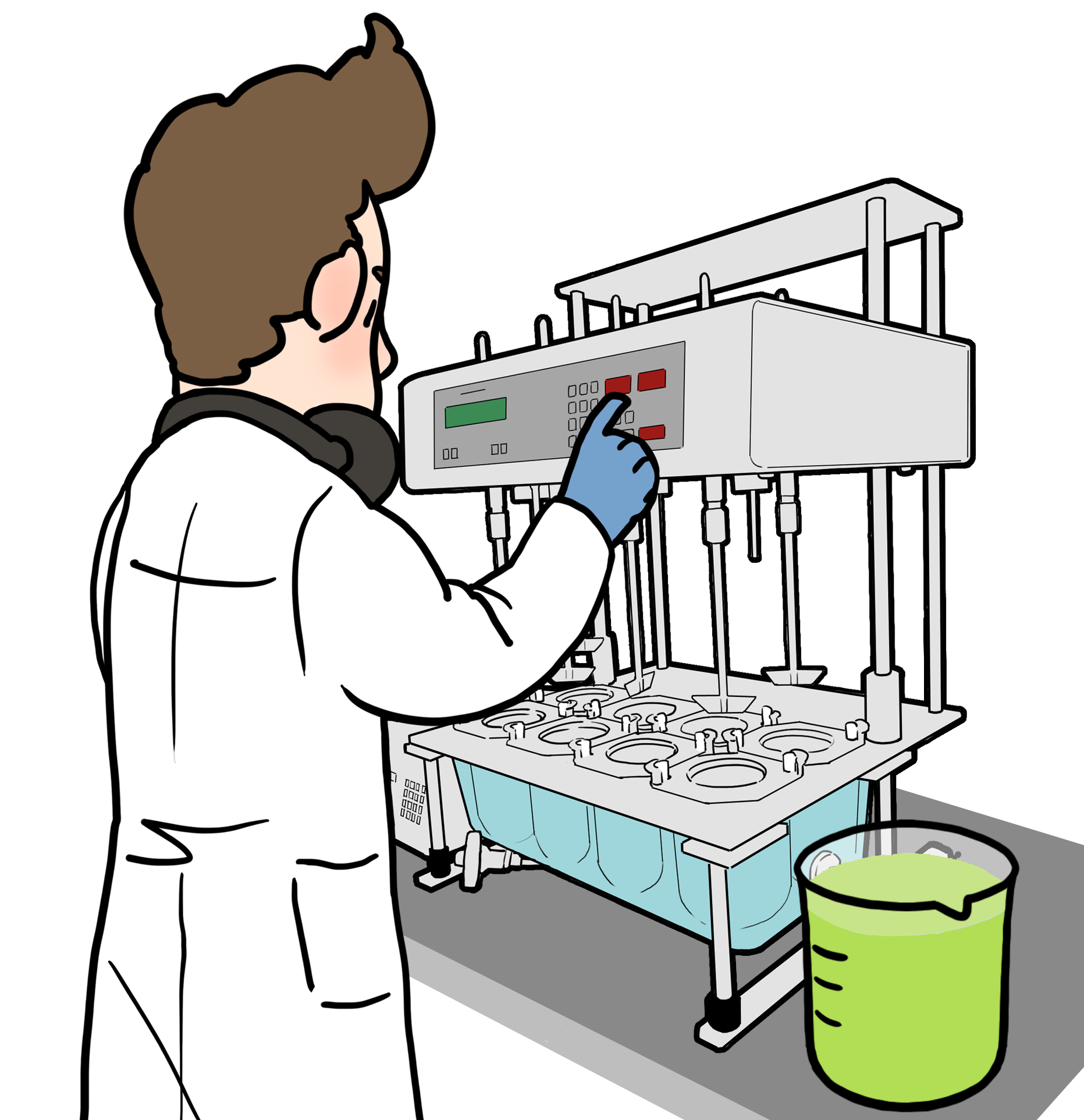
2) Carry out dissolution of the drug product when it first contacts FaSSGF (Fasted State Simulated Gastric Fluid) and then when it is converted into FaSSIF. This is known as 'two stage' biorelevant dissolution.
The value of these experiments is considerable. After a patient has taken their drug with a glass of water:
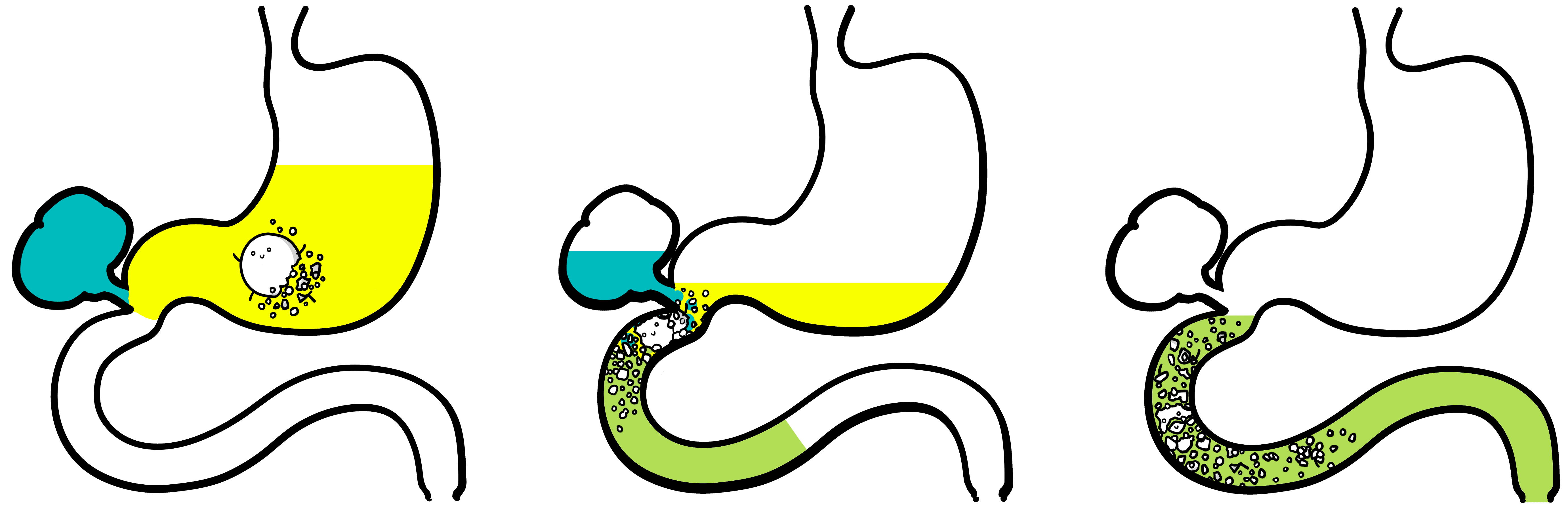
1) Drug dissolution starts in gastric fluid 2) Bile juice converts transferred gastric fluid... 3)...into small intestinal fluid. Drug dissolution continues
Biorelevant’s 'two stage' dissolution experiment simulates this in vivo transfer process. It is based on the USP two stage dissolution method but uses in vitro biorelevant media that simulate in vivo fasted state stomach (FaSSGF) and intestinal fluids (FaSSIF):

1) Drug dissolution starts in 450mL of FaSSGF 2) Adding 450mL of FaSSIF Converter... 3)...transforms it into FaSSIF. Drug dissolution continues
Which type of drug and formulation is this experiment suitable for?
The FaSSIF dissolution and two stage biorelevant dissolution experiments are particularly useful for poorly water-soluble basic drugs where absorption may be influenced by gastrointestinal drug supersaturation or precipitation. It is suitable for both immediate release and enteric coated formulations. These experiments help you to understand:
- Intestinal dissolution without the influence of gastric fluids
- Gastric drug dissolution and stability before and after the transfer process
- Potential gastrointestinal precipitation or supersaturation behaviour in simulated small intestinal fluid (FaSSIF)
- Results can reveal supersaturation or crystallisation behaviour within intestinal fluids from which absorption occurs to help monitor magnitude of supersaturation
- How these properties can influence and improve absorption of your drug
We have produced step-by-step guides to help you conduct drug analysis, filter adsorption, FaSSIF dissolution and two stage biorelevant dissolution which you can download below. These guides will help you standardize your experiments and achieve consistent results.
METHOD
MATERIALS


300mL is required of FaSSIF for drug analysis. We recommend running a dissolution test in FaSSIF (900mL/vessel) before the two stage biorelevant dissolution experiment. This is to understand drug dissolution in fasted intestinal fluids without interference from stomach fluids.

We recommend 300mL of FaSSGF for drug analysis. To simulate fasted state stomach fluid, 450mL of FaSSGF is required per vessel for the two stage biorelevant dissolution experiment.


FaSSIF Converter is used with 3F Powder to transform 450mL of FaSSGF into 900mL of FaSSIF.

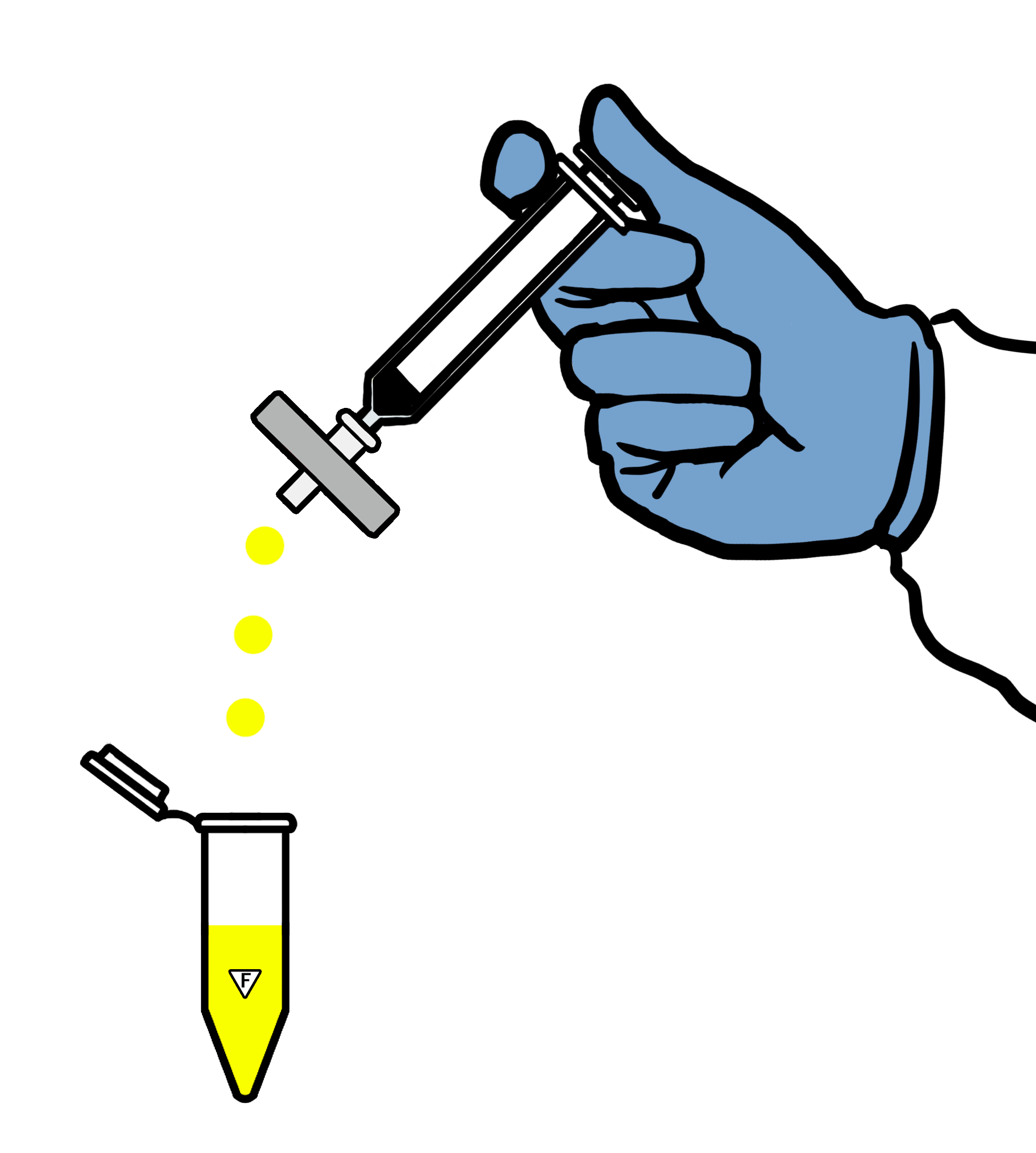
For optimal results, we strongly advise using a fresh filter at each sampling time point to halt the dissolution process. Our boxes of custom-made dissolution filters contain 72 pieces.
RECOMMENDED BUYS





We recommend 1 x FFF02, 1 x FASBUF, 1 x FASGBUF, 1 x 2ST-FASBUF and 1 x GMF-13.















































High filtration efficiency with minimal risk of clogging or bursting

Low drug adsorption and negligible extractables



























 Home
Home















