
Biorelevant Dissolution
-
Reveal how an oral drug will be released from a formulation and dissolve in simulated gastrointestinal fluids
-
Compare in vitro dissolution profiles of a Test Formulation against the Reference Formulation
-
Streamline preclinical development by linking your oral drug's in vitro biorelevant solubility and dissolution to in vivo preclinical bioavailability
-
Results help guide formulation development and optimization
Background
For an orally administered drug to be absorbed, it must first undergo dissolution; the drug must be released from its formulation and dissolve within fluids present in the gastrointestinal tract. The way a drug behaves in these fluids strongly influences how much drug is absorbed. To understand this in vivo process, simple in vitro dissolution tests using biorelevant media are performed. These in vitro tests use conventional dissolution apparatus and vessels containing biorelevant media to simulate the specific in vivo fluids found in different sections of the fasted and fed state gut.
Benefits of biorelevant dissolution experiments for new drug development
- Reveal how a formulated drug is likely to behave in vivo after oral administration under fasted and fed conditions
- Identify potential developability challenges such as poor absorption or food effects
- Guide the selection of an appropriate oral formulation strategy
Benefits of biorelevant dissolution experiments for generic drug development
Biorelevant media can be used for discriminatory dissolution studies which enable developers to:
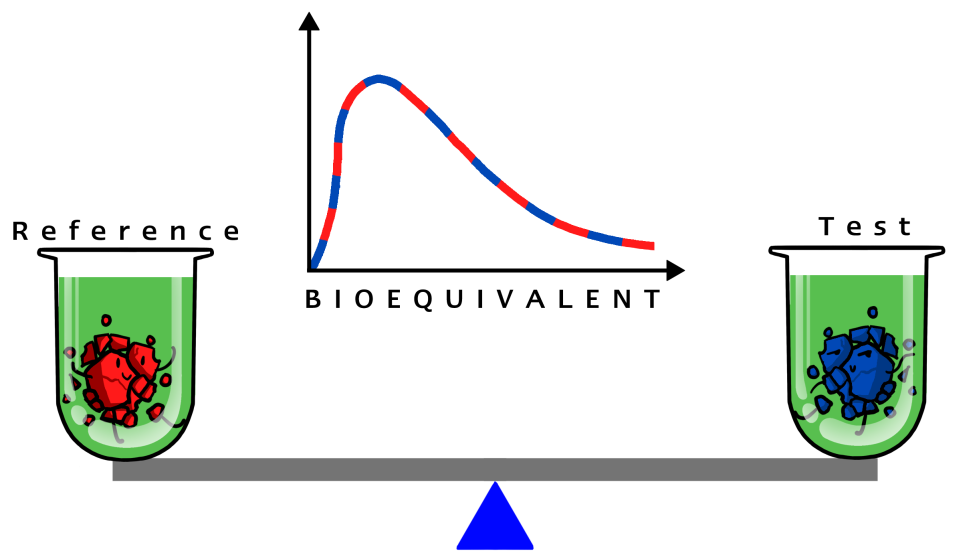
- Compare a Test Formulation against a Reference Formulation
- Detect significant in vitro differences in dissolution behaviour that could affect in vivo performance
- Optimize formulations in vitro before beginning costly in vivo bioequivalence studies thereby reducing the risk of failure and saving costs associated with failed bioequivalence studies
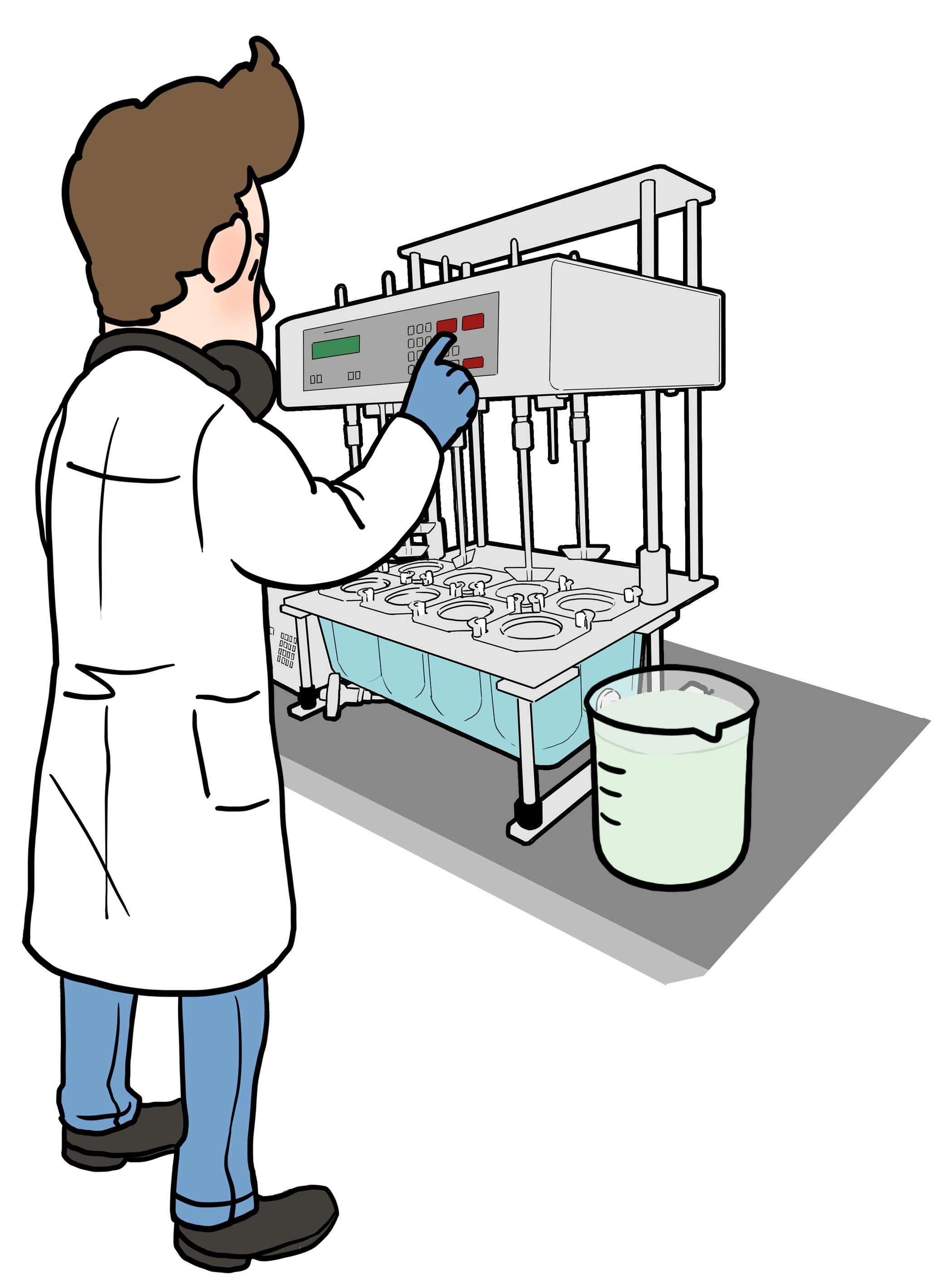

How to conduct the experiments
*Biorelevant dissolution: determine the rate and extent of drug release from the formulation into the biorelevant medium using USP Apparatus II (paddle method)
*Drug analysis: dilute biorelevant dissolution samples and analyse drug release with High-Performance Liquid Chromatography (HPLC)
*Data comparison: dissolution profiles of the Test and Reference Formulations are compared to assess similarity or detect differences that can affect drug absorption
Getting started
We have produced step-by-step guides to help you conduct drug analysis and biorelevant dissolution which you can download here:
METHOD
MATERIALS


FaSSIF is made with 3F Powder + FaSSIF Buffer. We recommend 300mL of medium for drug analysis and 900mL per dissolution vessel.


FeSSIF is made with 3F Powder + FeSSIF Buffer. We recommend 300mL of medium for drug analysis and 900mL per dissolution vessel.

FaSSGF is made with 3F Powder + FaSSGF Buffer. We recommend 300mL of medium for drug analysis and 900mL per dissolution vessel.


FaSSIF-V2 is made with FaSSIF-V2 Powder + FaSSIF-V2 Buffer. We recommend 300mL of medium for drug analysis and 900mL per dissolution vessel.


FeSSIF-V2 is made with FeSSIF-V2 Powder and FeSSIF-V2 Buffer. We recommend 300mL of medium for drug analysis and 900mL per dissolution vessel.

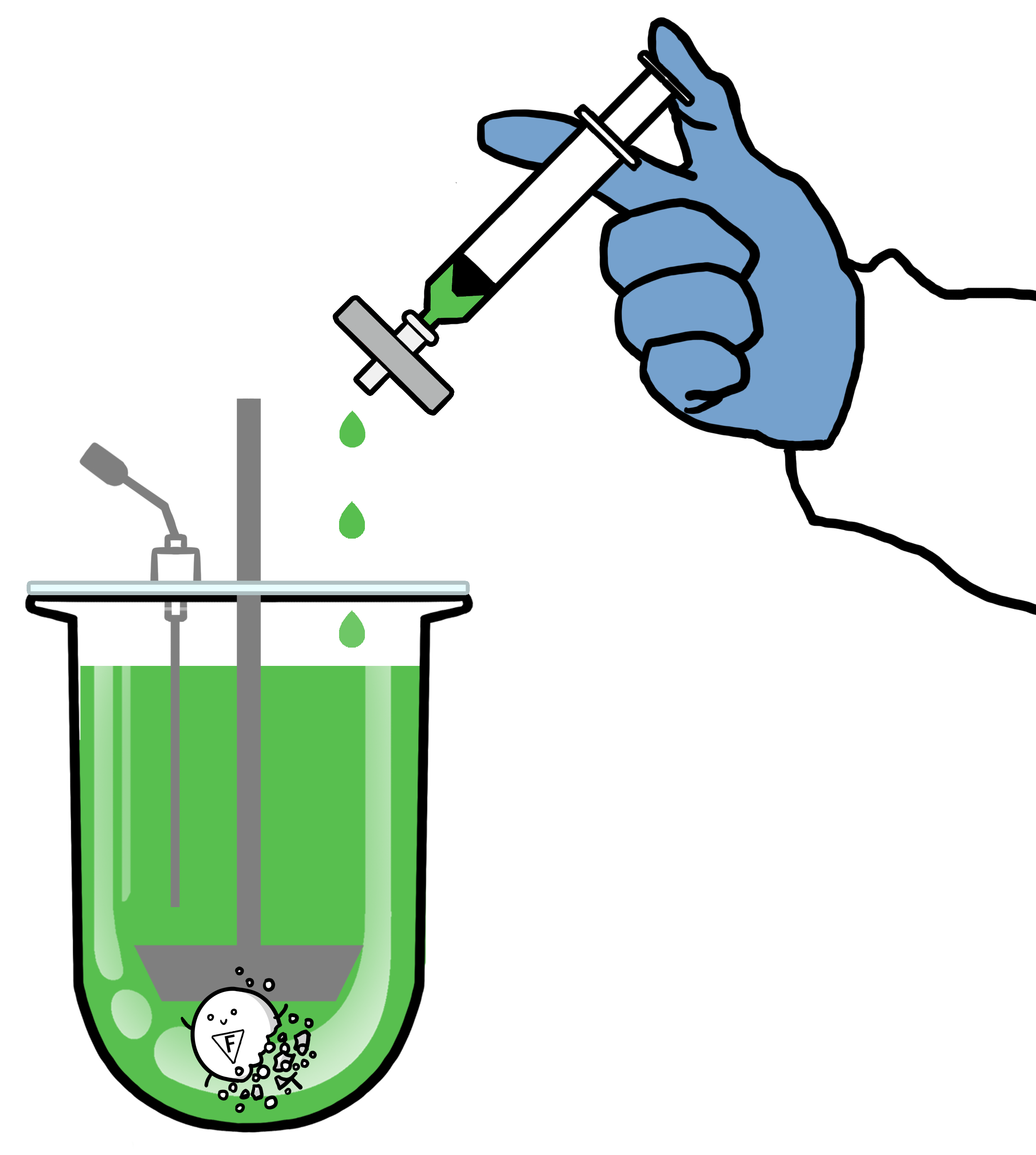
For optimal results, we strongly recommend using a fresh filter at each sampling time point to halt the dissolution process. Our boxes of dissolution filters contain 72 pieces.
SELECT MEDIUM

























High filtration efficiency with minimal risk of clogging or bursting

Low drug adsorption and negligible extractables








We recommend 1 x FFF02, 5 x FASBUF and 1 x GMF13
























High filtration efficiency with minimal risk of clogging or bursting

Low drug adsorption and negligible extractables









We recommend 1 x FFF02, 2 x FESBUF and 1 x GMF13
























High filtration efficiency with minimal risk of clogging or bursting

Low drug adsorption and negligible extractables









We recommend 1 x FFF01, 1 x FASGBUF and 1 x GMF13


























High filtration efficiency with minimal risk of clogging or bursting

Low drug adsorption and negligible extractables









We recommend 1 x V2FAS, 5 x V2FASBUF and 1 x GMF13























High filtration efficiency with minimal risk of clogging or bursting

Low drug adsorption and negligible extractables









We recommend 1 x V2FES, 1 x V2FESBUF and 1 x GMF13



















 Home
Home















