Biorelevant dissolution of Invokana® (canagliflozin) film coated tablets
Background
Canagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor used to treat type 2 diabetes. It lowers the risk of heart attack, stroke, or death in patients with type 2 diabetes and heart or blood vessel disease.
This post reveals how the BCS Class 2 drug canagliflozin behaves in BCS buffers and also intestinal biorelevant media. The Reference film coated tablets containing 300 mg of Canagliflozin were tested in the set of BCS buffers and then in the two biorelevant intestinal fasted ad fed state media FaSSIF and FeSSIF.
Only the reference was tested in this case study as the proprietary product is currently patent protected in EU markets.
Method
Dissolution profiles of the Reference determined using our Biorelevant Dissolution Guide. To enable a back-to-back comparison with BCS data both sets of dissolution tests (BCS and biorelevant) used the same parameters (n=6, 75rpm, 900 mL volume and 37°C). HPLC was used for analysis of canagliflozin as HPLC should be used for biorelevant drug analysis. An HPLC method was successfully developed for selectivity, linearity, precision, and accuracy. Samples were found to be stable for the duration of the analysis. The dissolution method was also found to be accurate and precise using a USP 2 apparatus.
BCS dissolution
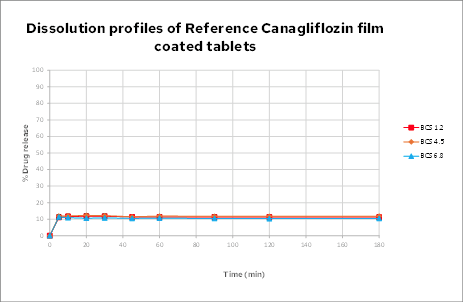
The overlapping BCS dissolution profiles confirm that the drug behaves as a neutral drug across the physiological pH of the gastrointestinal tract. The results also confirm the drug would be classed as insoluble (either BCS Class 2 or BCS Class 4) according to the BCS solubility categorization. Only about 10% of the drug is released from the drug product in across all three BCS media.
Biorelevant dissolution
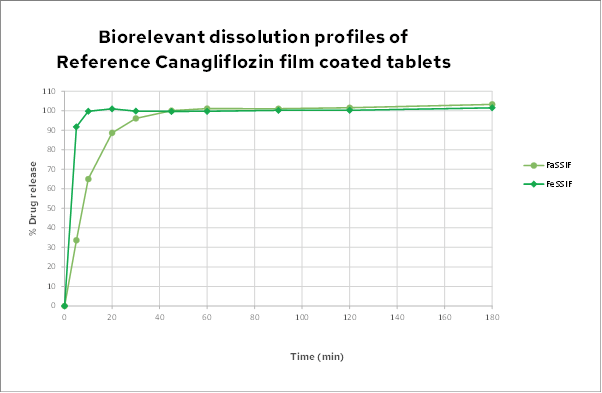
In sharp contrast to the BCS dissolution, the dissolution profiles in biorelevant media are markedly different from the BCS dissolution results. In both fasted (FaSSIF) and fed (FeSSIF) state media the dissolution of the complete dose is achieved. This dissolution behaviour is very rare for a moderate to high dose (300mg) BCS 2 neutral drug, especially in fasted state medium. It demonstrates that this insoluble drug has very significant interaction with biorelevant media.
Although FeSSIF shows potential as a dissolution medium, it would be far less appropriate than FaSSIF: This is because FeSSIF dissolution would be too fast and its discriminatory power would be insufficient.
High absorption and fast Tmax
The complete and fast dissolution profiles in biorelevant media explain why the oral absorption of this BCS Class 2 drug is high and the Tmax of the drug in the fasted state is rapid (ca 1.5 hours).
Discriminatory tool
FaSSIF is an ideal development tool to guide formulation development of either a bioequivalent generic test canagliflozin product (300 mg) or the fixed dose combination product containing canagliflozin with metformin. FaSSIF would provide the appropriate level of discrimination for your formulation development of these test products.



















 Home
Home















