Discriminatory dissolution potential of FEDGAS for Celecoxib 200mg hard gelatine capsules
This post shows the potential of FEDGAS as a biorelevant development dissolution medium when developing immediate release Test Products of the COX-2 inhibitor celecoxib. Celecoxib is neutral across the physiological pH of the gut so only one pH of FEDGAS medium was tested.
Dissolution profiles of Reference celecoxib capsules (200mg) were established in FEDGAS pH 6.0 and Biopharmaceutical Classification System (BCS) pH 6.8 medium.
Analytical and Dissolution Method
An HPLC method was successfully developed for selectivity, linearity, precision and accuracy. Samples were found to be stable for the duration of the analysis and a suitable sample filtration method was identified. The dissolution method was found to be accurate and precise using a USP 2 apparatus.
Dissolution Profiles
Both sets of dissolution tests used the same parameters (n=6, 75rpm, 900 mL volume and 37°C). The results are provided below.
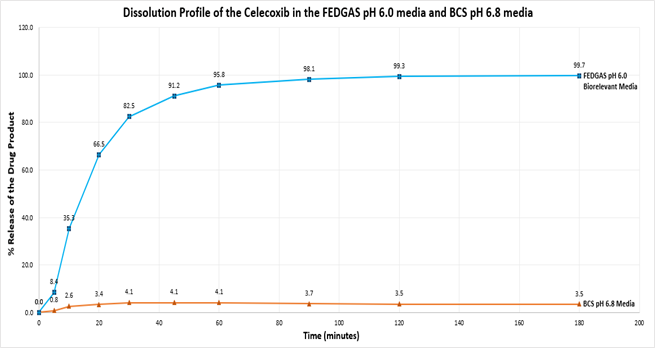
The graphs demonstrate clear differences between BCS pH 6.8 and FEDGAS pH 6.0 media. In BCS pH 6.8, just 4% of the dose is released. This low level of release is unsatisfactory for performance dissolution tests for celecoxib and reflects the water insolubility of this lipophilic drug. In contrast, 100% drug release is achieved in FEDGAS pH 6.0. This shows that FEDGAS is a good biorelevant medium to dissolve the complete 200 mg of Celebrex®. Additionally, the two to three hours required for complete dissolution allow for excellent discrimination if performing Comparative Dissolution Profile (CDP) studies of Test Product against the celecoxib Reference Product.
You can download the Dissolution guid here.



















 Home
Home















