Discriminatory dissolution potential of FEDGAS for Xarelto® (Rivaroxaban) 20 mg film-coated tablets
This post compares the potential of FEDGAS as a biorelevant development dissolution medium against the QC dissolution medium of Rivaroxaban. Rivaroxaban is a Xa inhibitor, neutral across the physiological pH of the gastrointestinal fluid so only one pH of FEDGAS (4.5) was tested. The 20 mg dose of Rivaroxaban has a positive food effect and should be taken with food.
Dissolution profiles of Reference rivaroxaban 20 mg film-coated tablets were established in FEDGAS Mid (pH 4.5) and QC release Acetate buffer pH4.5 + 0.4% sodium dodecyl sulphate (SDS) medium.
Analytical and Dissolution Method
An HPLC method was successfully developed and assessed for selectivity, linearity, precision and accuracy. Samples were found to be stable for the duration of the analysis and a suitable filtration approach was identified. The dissolution method was found to be accurate and precise using a USP II apparatus. Both dissolution tests used the same parameters as outlined in Biorelevant dissolution guide.
Dissolution Results
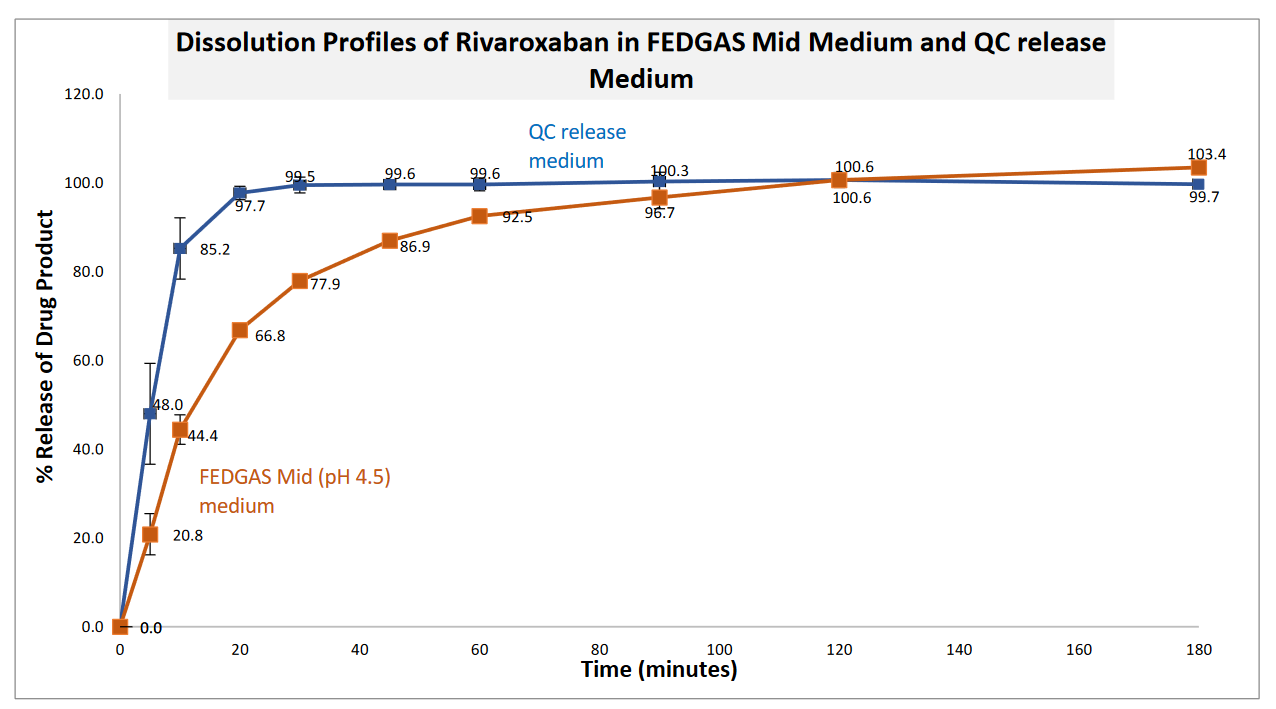
The graphs reveal a significant difference between the QC release and FEDGAS Mid (pH 4.5) medium. Using the QC release method, 85% of dose was released in just 10 minutes. This rapid dissolution rate (due to the presence of SDS) will not provide adequate discrimination between the Reference and a Test formulation. In sharp contrast, FEDGAS Mid medium released the drug in a more gradual and smooth way. FEDGAS will be an effective biorelevant medium to provide discrimination between the 20 mg of film coated tablets of Xarelto® and its Test Product. The two to three hours required for complete dissolution of the Reference will allow for excellent discrimination when performing Comparative Dissolution Profile (CDP) with a Test Product. You can download the guide here.



















 Home
Home















