
Fed Gastric (FEDGAS) Dissolution
-
Simulate dissolution of a drug product in different stage stomach fluids (early, mid, late) after intake of an FDA high-fat meal
-
Particularly important for drugs where absorption is positively affected by increased solubility due to the presence of fats
-
Check how a drug behaves in the stomach during digestion as changing pH levels due to stomach emptying can affect a drug's metabolism and absorption rate
-
For drugs that exhibit a positive food effect, the experiments can be discriminatory and reveal potential differences in absorption between formulations
Background
The composition of fed state stomach fluid changes in vivo over time as digestion of an FDA high-fat meal takes place. This high-fat meal is used in human clinical studies to control the calorific content of the food co-administered when testing a drug product so the digestive physiological response to the food is standardized. One of the most notable changes in the fluid is the decrease in pH which facilitates digestion. Three different FEDGAS dissolution media (Early, Mid and Late) simulate this process. These media are filterable allowing dissolved drug to be readily separated from undissolved particles using 0.45μm pore size filters. FEDGAS media also simulate the levels of carbohydrates and fats present in the standard high-fat FDA meal.
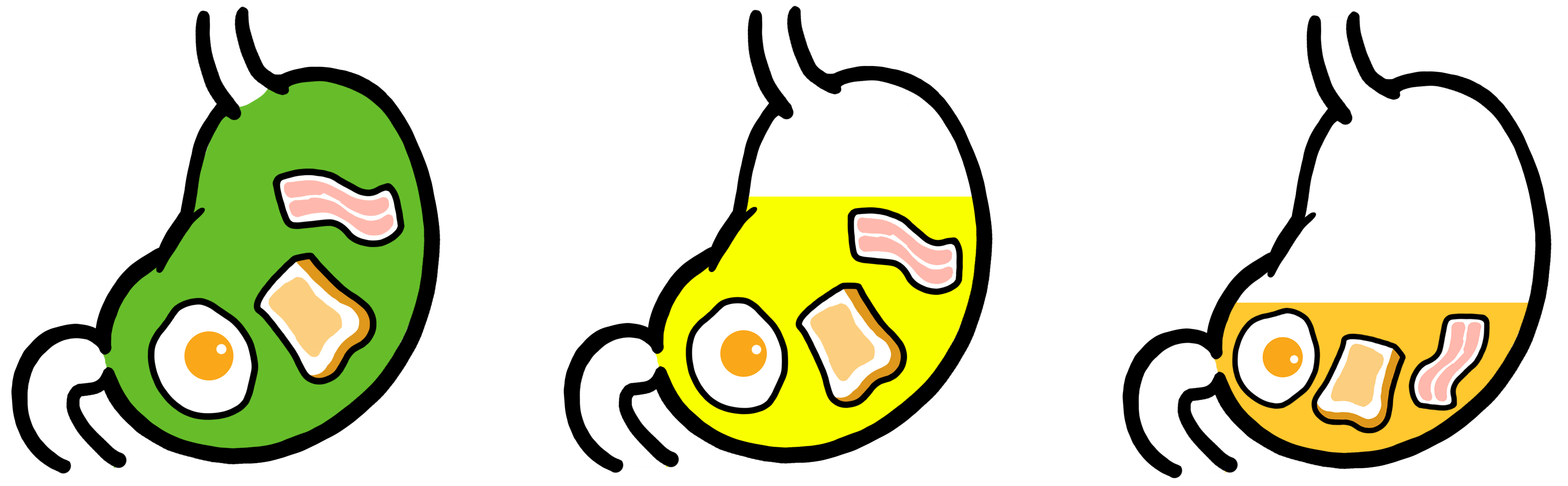
FEDGAS Early (pH6) FEDGAS Mid (pH4.5) FEDGAS Late (pH3)
Which type of drugs are these experiments most useful for?
FEDGAS biorelevant dissolution experiments are particularly useful for poorly water-soluble basic drugs whose absorption may be enhanced by co-administration with a high-fat meal. It is suitable for both immediate-release and enteric-coated formulations.
Which FEDGAS dissolution media should I test my drug in?
*For pH sensitive drugs: testing across all three FEDGAS media (Early, Mid and Late) provides insight into how dissolution is affected over time, helping to identify the most discriminatory medium for your formulation.
*For neutral drugs: dissolution testing in a single FEDGAS medium is usually sufficient because pH has limited influence on solubility.
*If the formulation includes pH sensitive excipients or coatings (for example modified-release products), testing across all three FEDGAS media is recommended.

We have produced step-by-step guides to help you conduct drug analysis, filter adsorption studies and FEDGAS dissolution testing. These guides can help you standardize your experiments and achieve consistent, reproducible results.
METHOD
MATERIALS


FEDGAS Early is made with FEDGAS Gel + FEDGAS Buffer Concentrate pH6. We recommend using 300mL of the medium for drug analysis and 900mL per dissolution vessel.


FEDGAS Mid is made with FEDGAS Gel + FEDGAS Buffer Concentrate pH4.5. We recommend using 300mL of the medium for drug analysis and 900mL per dissolution vessel.


FEDGAS Late is made with FEDGAS Gel + FEDGAS Buffer Concentrate pH3. We recommend using 300mL of the medium for drug analysis and 900mL per dissolution vessel.

For optimal results, we strongly advise using a fresh filter at each sampling time point to halt the dissolution process. Our boxes of custom-made dissolution filters contain 72 pieces.
RECOMMENDED BUYS





We recommend 3 x FDG-GEL, 1 x FDG-BUF6, 1 x FDG-BUF4.5, 1 x FDGD-BUF3 and 1 x GMF13.

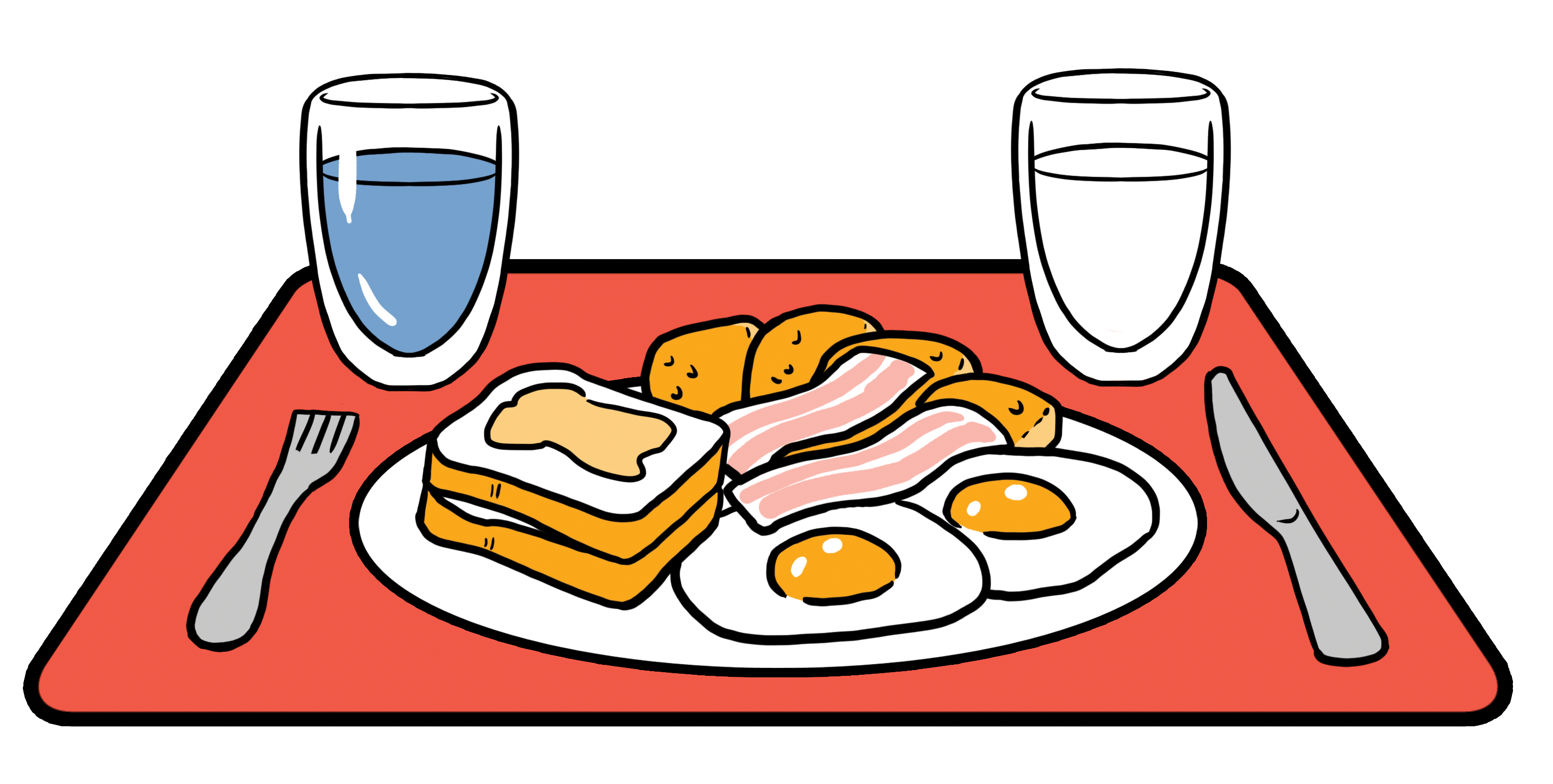
900mL of FEDGAS contains the 62.5g of fat present in a high-fat FDA meal along with the bile salts present in the fed stomach














































High filtration efficiency with minimal risk of clogging or bursting

Low drug adsorption and negligible extractables



























 Home
Home















