Biorelevant dissolution test method
We have developed a biorelevant dissolution test method that is very easy to follow and uses standardized USP 1 or 2 apparatus. It can be used for most immediate release oral Test and Reference formulations. We routinely use this method in our laboratory. The test is based around USP Chapter <1092> 'The Dissolution Procedure' but it's specifically designed for BCS Class 2 drugs in any Biorelevant Media. If you have an existing QC HPLC method, this can typically be used or adapted to analyse your drug.
The guide is in two parts:
HPLC method evaluation
In the first section, we recommend conducting a few preliminary HPLC assessment experiments before dissolution testing and analysis of your dosage form. Once these pre-experiments have been completed, dissolution tests can then be carried out.
Dissolution test
The second section of our guide uses parameters of the existing dissolution method you may have such as the use of a sinker and mixing speed. However, it also contains useful guidance on media preparation, recommended volumes, sampling times as well as tips to minimize experimental variability. This should help you achieve highly reproducible results for your Test Formulation and Reference tested in any of our Biorelevant Media.
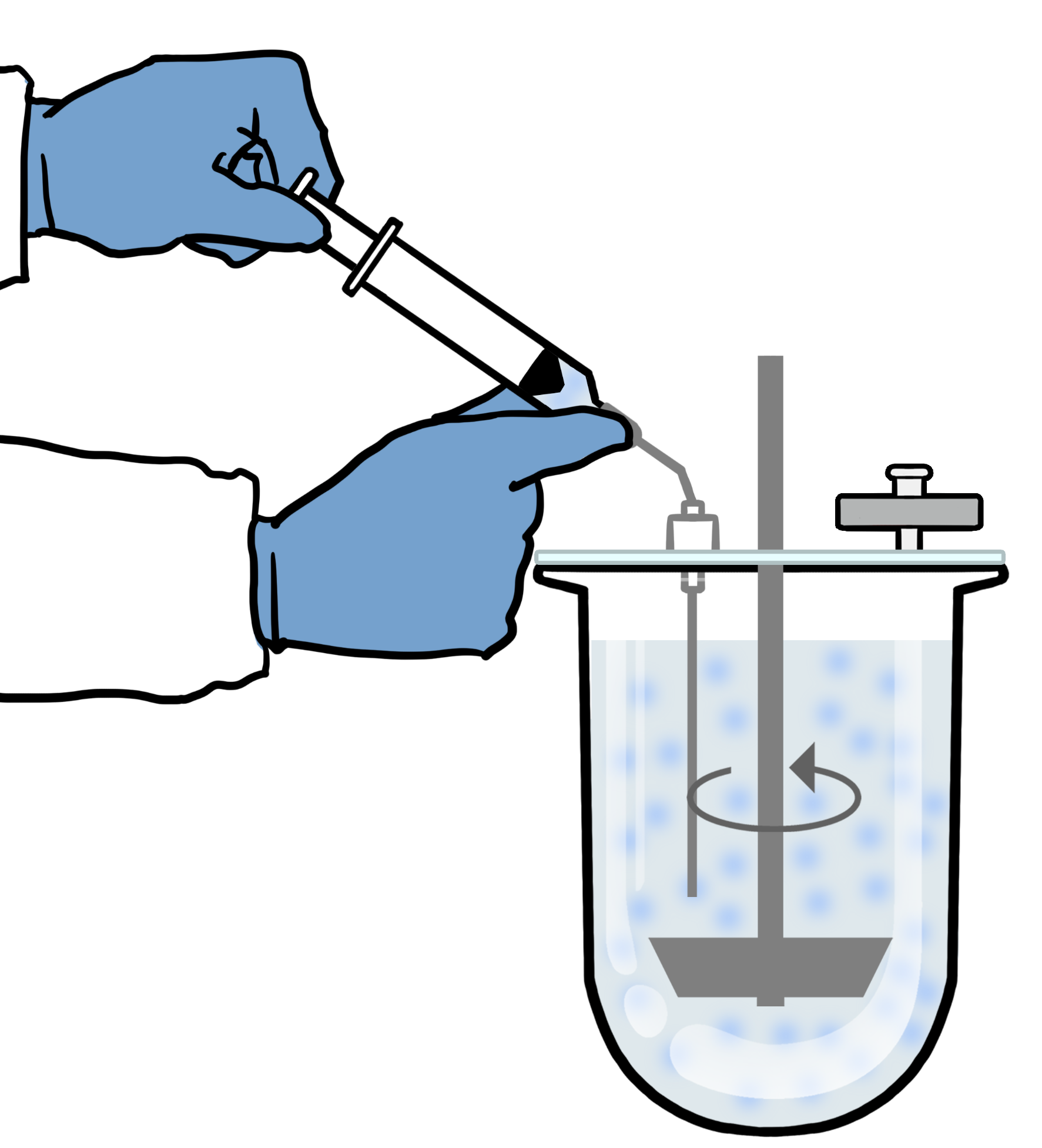
Please visit the following webpage for more information on biorelevant dissolution.



















 Home
Home















