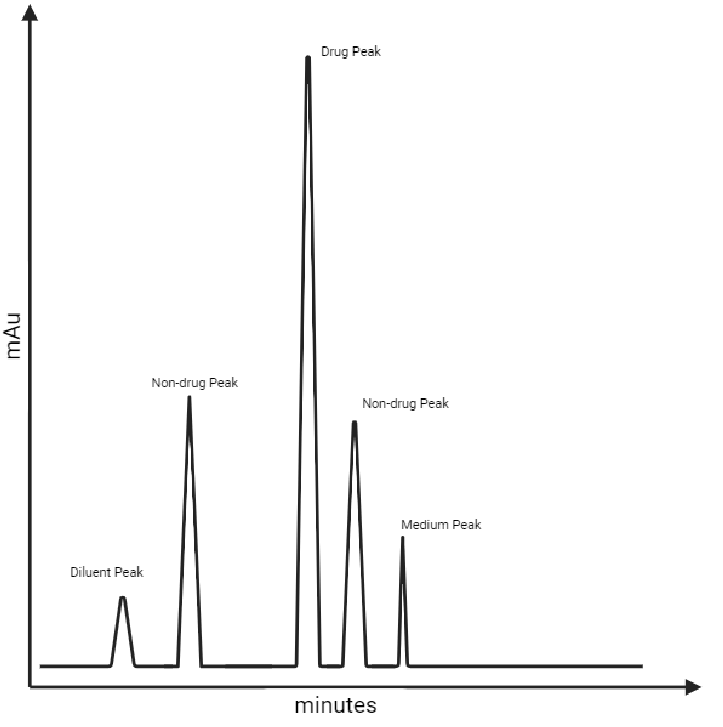
What is 'specificity'?
The capability to determine the drug in the presence of components (typically impurities, excipients and other matrix (dissolution medium) peaks) that could be present is known as 'specificity'. 'Specificity' is an integral part of analytical method validation and must be performed by carrying out multiple injections to eliminate potential errors that can occur during experimental sequence. Typically, other chromatographic properties such as peak area, retention time and tailing factor etc should also be assessed.
Download our Biorelevant Dissolution Guides here.



















 Home
Home















