pH reproducibility of two stage biorelevant dissolution kit
Two stage biorelevant dissolution involves testing the dissolution of a formulation of a basic drug in a USP 2 dissolution apparatus.
The media for the experiment can be reproducibly prepared using our Two Stage Biorelevant Dissolution Kit. Details for the preparation of the media can be found in the Two Stage Biorelevant Dissolution Guides. An overview of the two stage biorelevant dissolution method is provided below:
Buffer Concentrates
Buffer Concentrates have been developed not only to simplify media preparation but also to improve reproducibility.
Every batch of buffer concentrate is manufactured accurately and precisely using high quality components and a well-defined protocol. Every batch is also analysed to ensure it meets the required specifications.

Testing
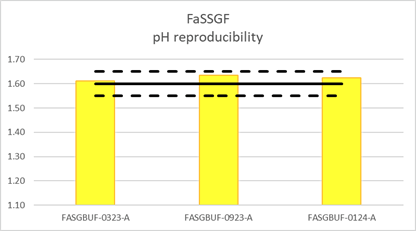
The pH of three different batches of FaSSGF was measured before addition of FaSSIF Converter.
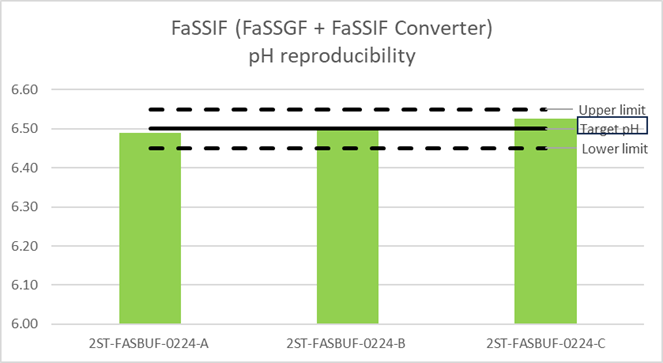
Similarly three independent batches of FaSSIF Converter were prepared using the two stage buffer concentrate.
The pH of FaSSIF (prepared by adding FaSSIF Converter to FaSSGF) was measured. All pH’s were measured at room temperature after 48hours of preparing the media. The results are provided below:
Results
After 48h at room temperature, as can be seen from the graphs above, the pH of both dissolution media prepared remained stable, within ±0.05pH units.
These Buffer Concentrates allow the preparation of highly accurate and precise media for two stage biorelevant dissolution testing.



















 Home
Home















