Two stage biorelevant dissolution
Two stage biorelevant dissolution is also referred to as a “biorelevant transfer test”. The method is designed to test how a drug product behaves when in contact with simulated gastric fluid (FaSSGF) which is subsequently converted into simulated intestinal fluid (FaSSIF). This in vitro experiment simulates the in vivo process when fasted stomach fluid containing a drug product is converted to fasted intestinal fluid.
Similar to USP method
It is a similar principle to the USP two-tier dissolution test described in USP Chapter <711> (Method A Procedure). However, the significant difference is that the acid and buffer stages used are biorelevant.
When to conduct two stage biorelevant dissolution
The test is mainly used during the development of drug products of water insoluble bases where solubility of the drug is higher in the gastric fluid than the intestinal fluid. The results reveal insights into precipitation or supersaturation as the pH is shifted from stomach to intestinal pH.
Equipment
For immediate release formulations of basic drug, the two stage biorelevant dissolution test is conducted in USP 2 apparatus.
The experiment
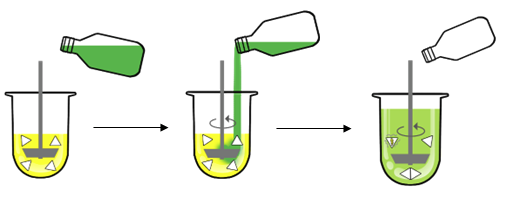
Two media need to be prepared: FaSSGF (simulating the stomach) and Concentrate FaSSIF. Dissolution is first carried out in FaSSGF (left side) and then continued after adding (middle image) Concentrated FaSSIF to FaSSGF to prepare FaSSIF (right Side image).
Carry out FaSSIF Dissolution
Before carrying out a two-stage biorelevant test, we strongly recommended first testing dissolution in FaSSIF (i.e. simulated fasted state intestinal fluid) to benchmark how the drug product is releasing prior to exposure to fasted stomach fluid.
This is particularly important for formulations of basic drugs with low water solubility and will allow you to assess the role of the stomach dissolution to create a supersaturated form of the drug (or not) in the small intestinal fluid.
Build Your Experiment
Step 1: Download and read Two Stage Biorelevant Dissolution Guide
Step 2: Use the Media Prep Tool to choose your volumes
Step 3: Build your experiment
















 Home
Home















