What is a High-Fat FDA meal?
A high-fat FDA meal is a standardized meal that is orally administered along with a drug product to create fed state/condition in human clinical trials.
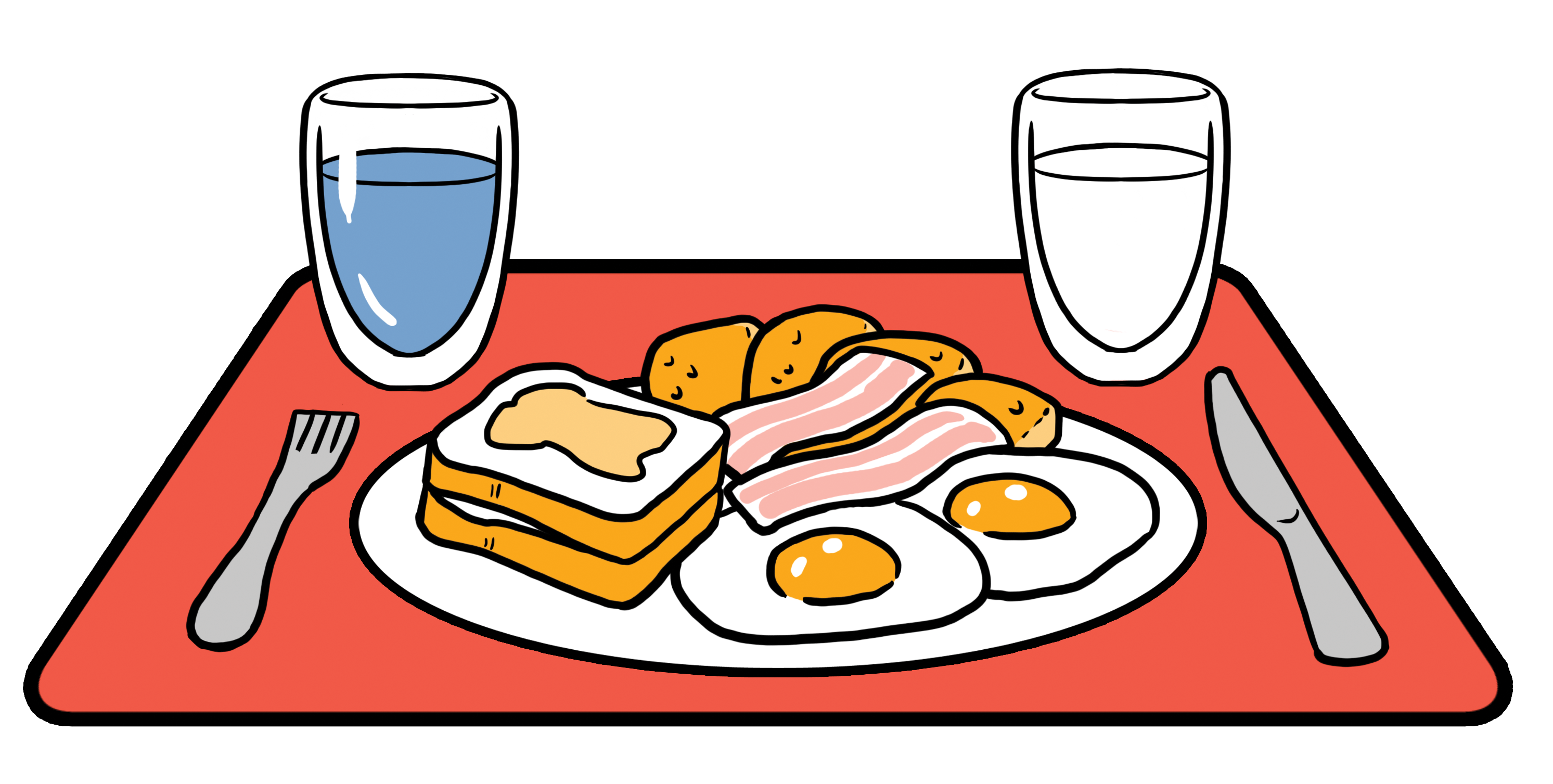
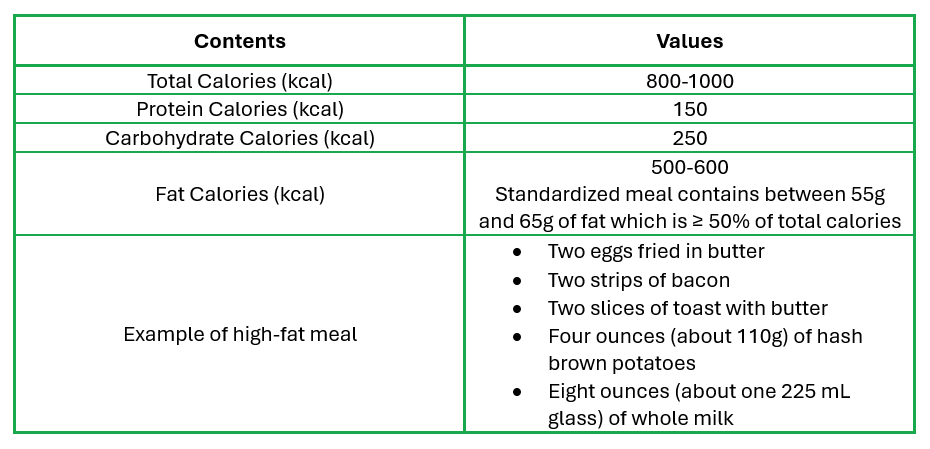
Composition of high-fat meal
Purpose of high-fat meal
This meal elicits a strong physiological digestive response within the gastrointestinal tract. Intake of this meal enables the impact of high calorific food on the pharmacokinetics of a drug product to be examined in a well-controlled manner. The high-fat content of the meal can significantly change the way a food-drug interaction occurs in the stomach and intestines.
There are different reasons for exploring administration of drug product with a high-fat meal:
• Food can help improve the tolerability of drugs by reducing localized gastric irritation of some drugs e.g. naproxen
• Improve the absorption of an insoluble drug typically by increasing drug solubility and dissolution e.g. rivaroxaban tablets 20mg
• Food may increase adversely e.g. nilotinib, decrease e.g. indinavir sulphate, or have no effect on the systemic drug level in a patient e.g. canagliflozin
Clinical usages of high-fat meal
Usages of this meal in clinical studies are referenced in various FDA draft Guidance for Industry:
• Bioavailability Studies Submitted in NDAs or INDs (April 2022)
• Assessing the Effects of Food on Drugs in INDs and NDAs (June 2022)
• Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA (DRAFT August 2021)
In vitro high-fat meal
FEDGAS (Early/Mid/Late) media are in vitro media that simulate the different stages of in vivo fed state gastric fluids by replicating the fat and carbohydrate composition of a high-fat meal. FEDGAS (Early/Mid/Late) media media help developers to examine and understand food-effect interactions (based on dissolution and solubility) using standard USP dissolution apparatus and HPLC analysis.
















 Home
Home















