Gefitinib two stage dissolution comparison (Biorelevant vs Non-biorelevant)
Introduction
The purpose of this study was to compare two stage biorelevant dissolution (stomach to intestinal fluid) experiment with an adapted two two-tier dissolution test described in USP <711>. The two-tier test involved converting an acidic stage (stomach pH) to intestinal pH with traditional phosphate buffer (non-biorelevant) without the mixed micelles present in intestinal fluids. In both cases the resultant pH of final medium after converting the FaSSGF into intestinal stage was pH6.5. Tablets of the tyrosine kinase inhibitor gefitinib (dose 250mg) was selected as the dosage form for this study. Gefitinib, the active ingredient is a basic compound with low water solubility. The dissolution and precipitation/supersaturation behaviour after this pH transition are critical to understanding the absorption and biopharmaceutical properties for this drug.
Materials
- Iressa® (12 x Gefitinib 250 mg tablets)
- 3F Powder® (2 x FFF01)
- FaSSGF Buffer Concentrate (FASGBUF)
- FaSSIF Converter Buffer Concentrate (FASCONV)
- GMF 13mm Filters 13mm (GMF13)
Methods and equipment
- Analytical Method: An HPLC method was validated, and linearity was performed by using online calculators
- Two stage dissolution guide
- Dissolution apparatus: USP 2
Media
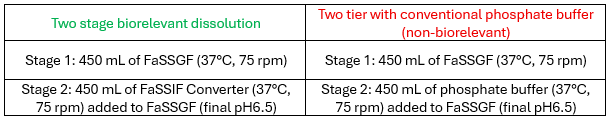
- Duration: 1 hour in FaSSGF followed by 2 hours in the intestinal stage medium
- Vessels: n = 6 x 2
- Sampling and Filtration: 13 mm Glass Microfibre Syringe Filters (fresh filter at each time point) were used to stop further dissolution and avoid artificial solubility enhancement in the intestinal stage
Results
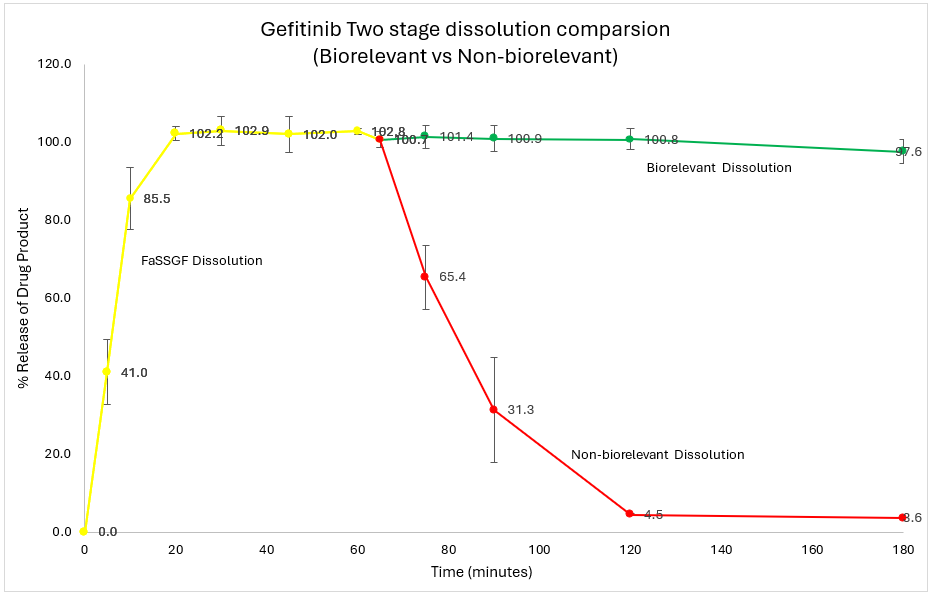
Gefitinib was released and completely dissolved during the first hour in FaSSGF.
Upon transition to FaSSIF, no significant decrease in gefitinib concentration was observed, despite the pH shift from acidic to near neutral. Gefitinib remained in a metastable supersaturated state throughout the two-hour intestinal stage of dissolution, without evidence of precipitation.
However, in sharp contrast, upon transitioning to the traditional phosphate buffer, gefitinib rapidly precipitated in the non-biorelevant medium and was not maintained in a supersaturated state.
Discussion
The dissolution profiles revealed that gefitinib can effectively dissolve in an acidic environment of the stomach. The subsequent maintenance of a supersaturated state in FaSSIF indicates that gefitinib, once solubilized in the stomach, does not readily precipitate upon entering the small intestine, and may, therefore, remain available for absorption.
Using traditional phosphate buffer in the two-stage dissolution of gefitinib may lead to misleading results, as it tends to results in rapid drug precipitation and does not reveal drug supersaturation. This is because the phosphate buffer lacks the physiological components present in biorelevant medium that help maintain the drug in a supersaturated state, simulating conditions found in vivo.
Physiological Relevance
Conducting two-stage dissolution using biorelevant media provides physiologically relevant conditions under which drug supersaturation, precipitation behaviour, and absorption potential can be effectively observed and evaluated.



















 Home
Home















