Two stage biorelevant dissolution of Stugeron® (cinnarizine 15 mg)
 Introduction
Introduction
Two stage biorelevant dissolution testing simulates the physiological transition of oral dosage forms from the acidic environment of the stomach to the near-neutral pH of the small intestine, providing insight into drug solubility and stability under gastrointestinal (GI) conditions. Cinnarizine, the active ingredient in Stugeron® (15 mg), is a basic compound with low water solubility, making it crucial to assess its dissolution behaviour across this pH transition. This study investigates cinnarizine's dissolution in simulated fasted-state gastric fluid (FaSSGF) and following conversion into fasted-state intestinal fluid (FaSSIF), with a focus on the potential for supersaturation during GI transit.
Materials
- Stugeron® (15 x Cinnarizine 15 mg tablets)
- 3F Powder®
- FaSSGF Buffer Concentrate (FASGBUF)
- FaSSIF Converter Buffer Concentrate (FASCONV)
- GMF 13mm Filters 13mm (GMF13)
Methods
- Two stage dissolution guide
- Dissolution apparatus: USP 2
- Media:
- Stage 1: 450 mL of FaSSGF (37°C, 75 rpm)
- Stage 2: 450 mL of FaSSIF Converter (37°C, 75 rpm) added to FaSSGF
- Duration: 1 hour in FaSSGF followed by 2 hours in FaSSIF
- Vessels: n = 6
- Sampling and Filtration: 13 mm Glass Microfibre Syringe Filters (fresh filter at each time point) were used to stop further dissolution and avoid artificial solubility enhancement.
- Analytical Method: An HPLC method was validated for selectivity, linearity, precision, and accuracy. Sample stability was confirmed for the duration of analysis.
Results
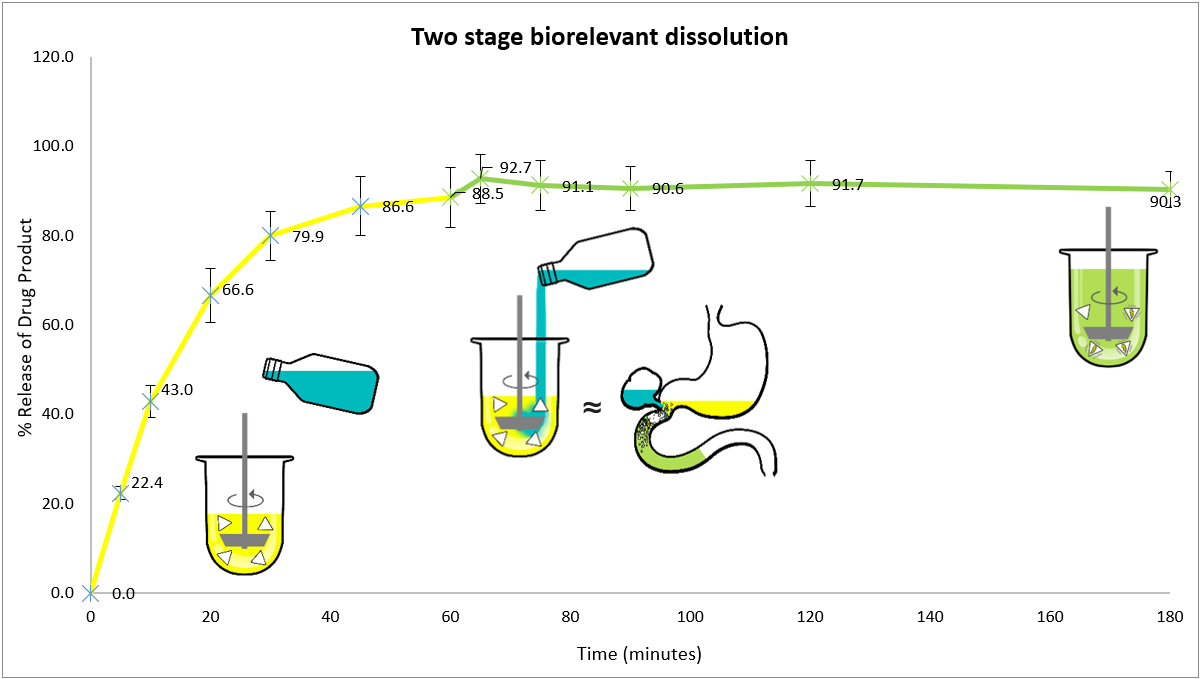
- Cinnarizine was released and dissolved during the first hour in FaSSGF.
- Upon transition to FaSSIF, no significant decrease in cinnarizine concentration was observed, despite the pH shift from acidic to near-neutral.
- The drug remained in a metastable supersaturated state throughout the two-hour intestinal stage of dissolution, without evidence of precipitation.
Discussion
The dissolution behaviour suggests that cinnarizine effectively dissolves in the acidic environment of the stomach. The subsequent maintenance of a supersaturated state in FaSSIF indicates that cinnarizine, once solubilized in the stomach, does not readily precipitate upon entering the small intestine, and may therefore remain available for absorption.
Physiological Relevance
Given the sustained supersaturation over two hours in intestinal conditions, the cinnarizine dissolved in the fasted-state stomach will likely be readily absorbed upon reaching the small intestine. This underscores the importance of gastric dissolution in the oral bioavailability of cinnarizine and supports the application of two-stage biorelevant dissolution testing as a predictive tool for in vivo drug performance.



















 Home
Home















