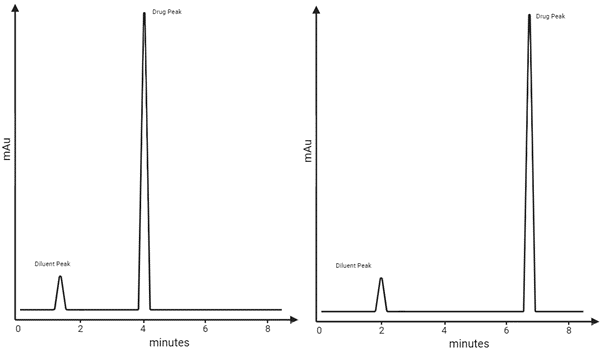
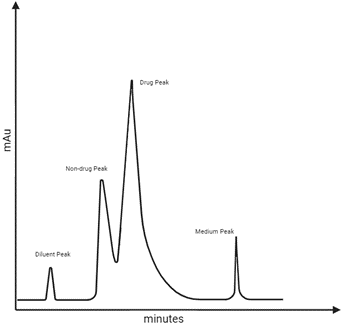
Learning Centre
ALL
NEW!
Background
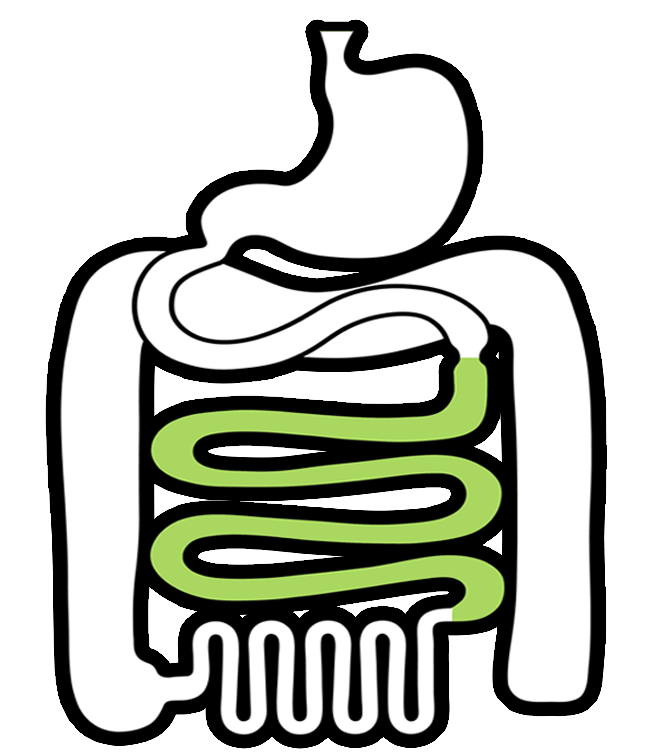
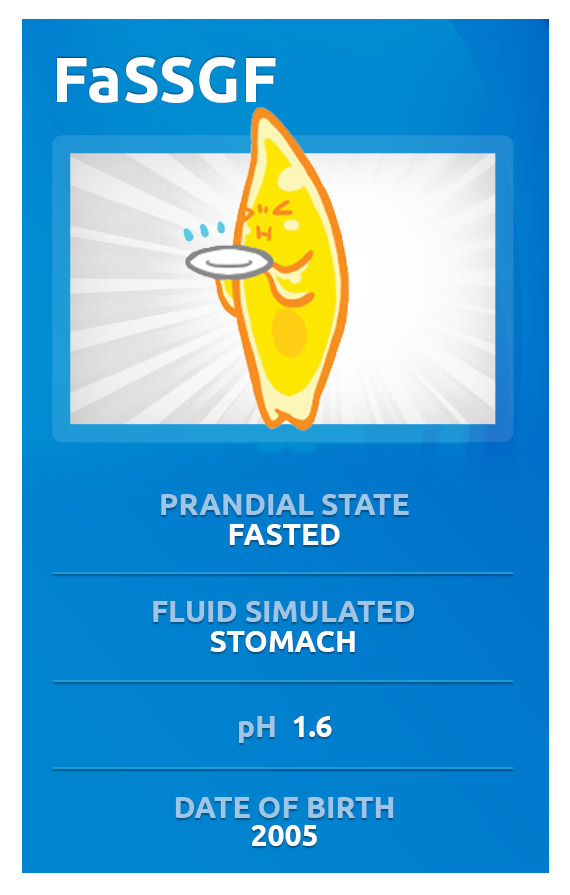
Biorelevant media
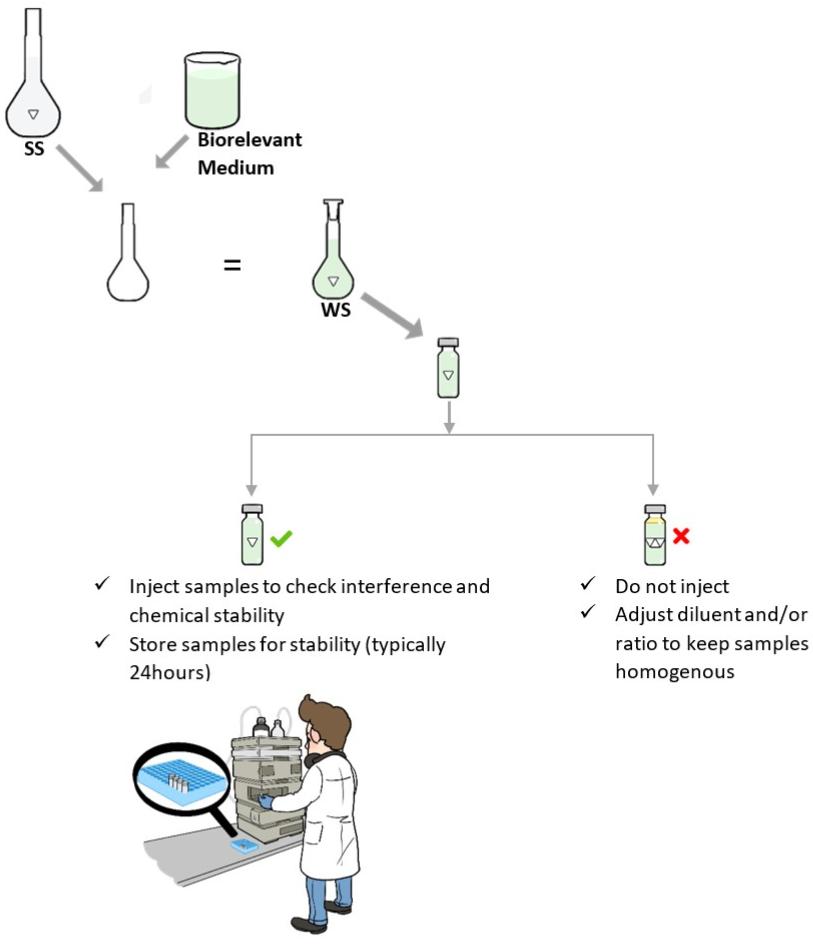
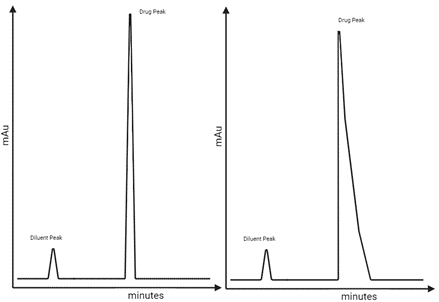
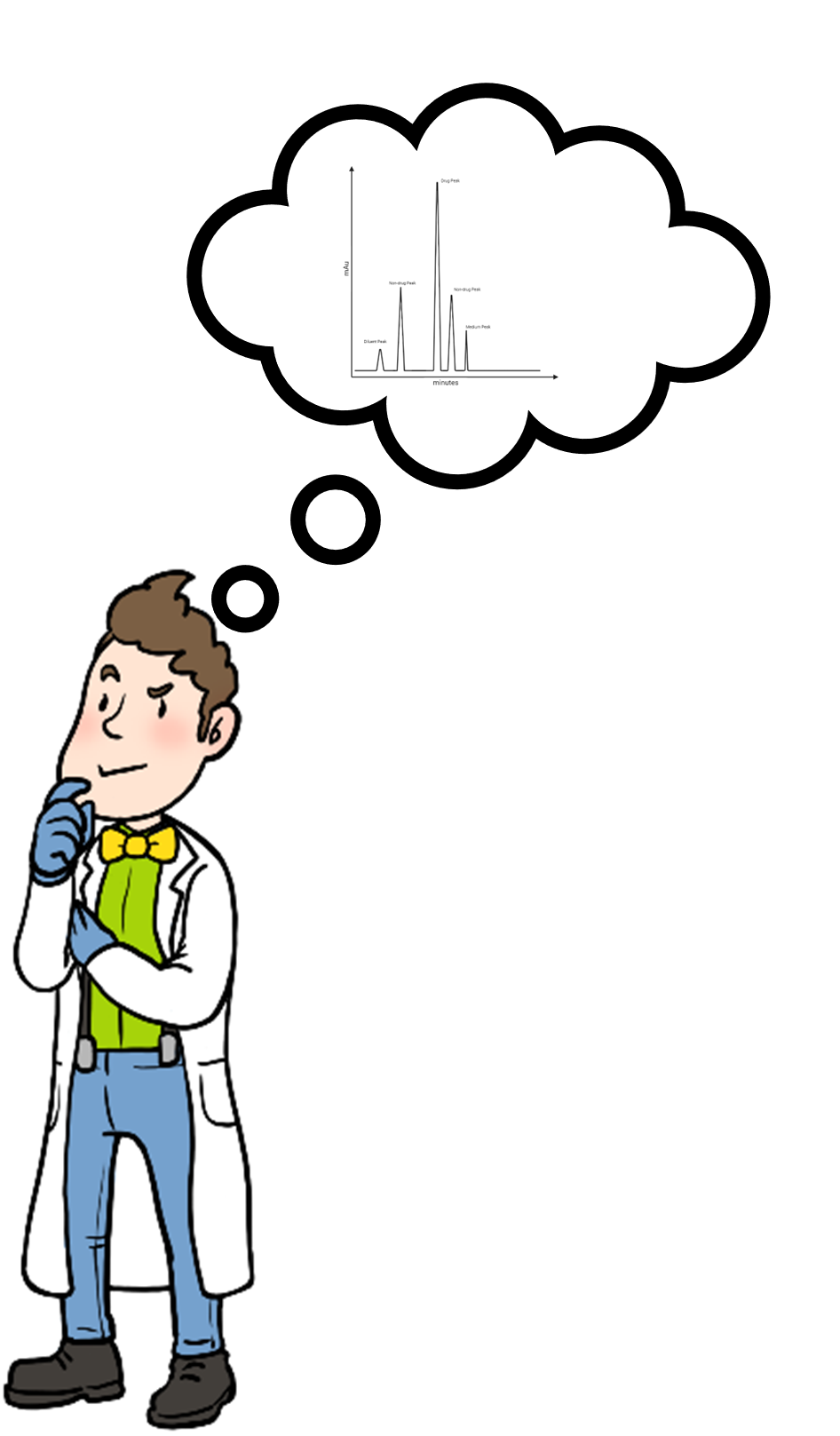
Analysis
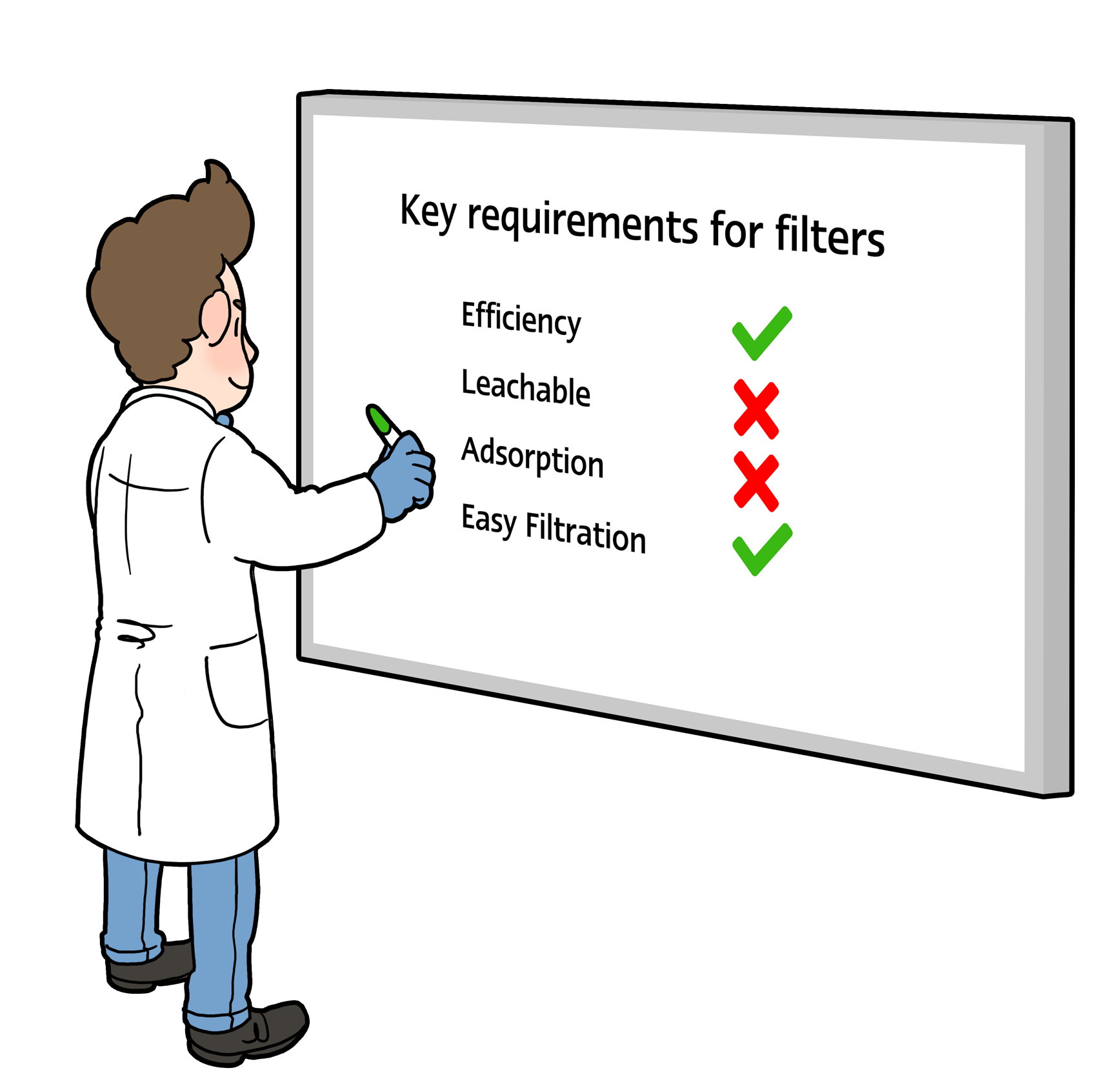
Filters
Dissolution tips
Guides and Calcs
Dissolution
















 Home
Home