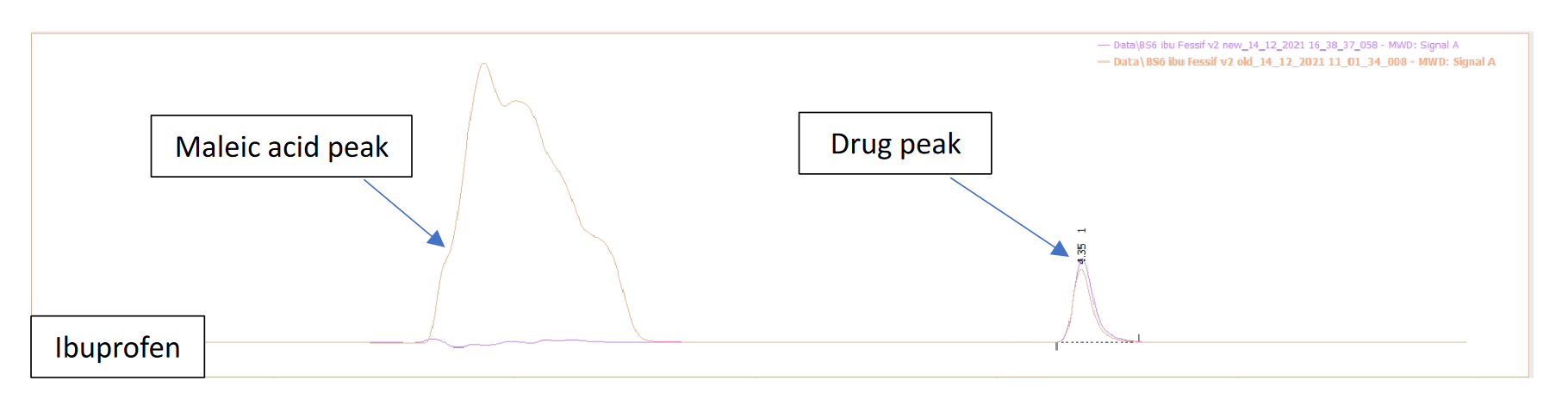
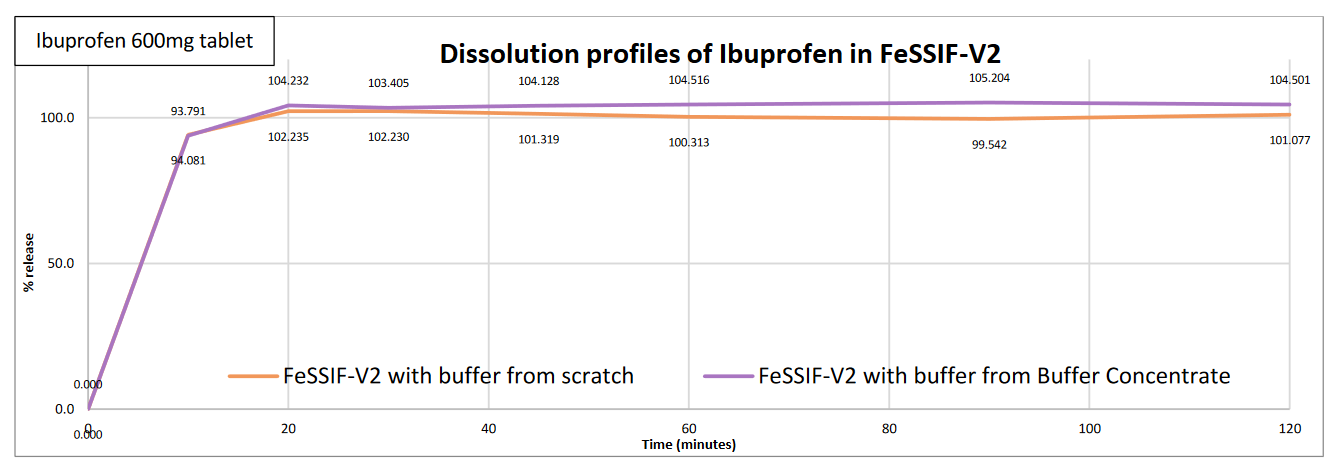
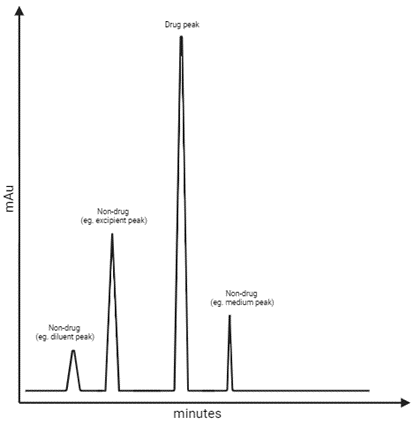
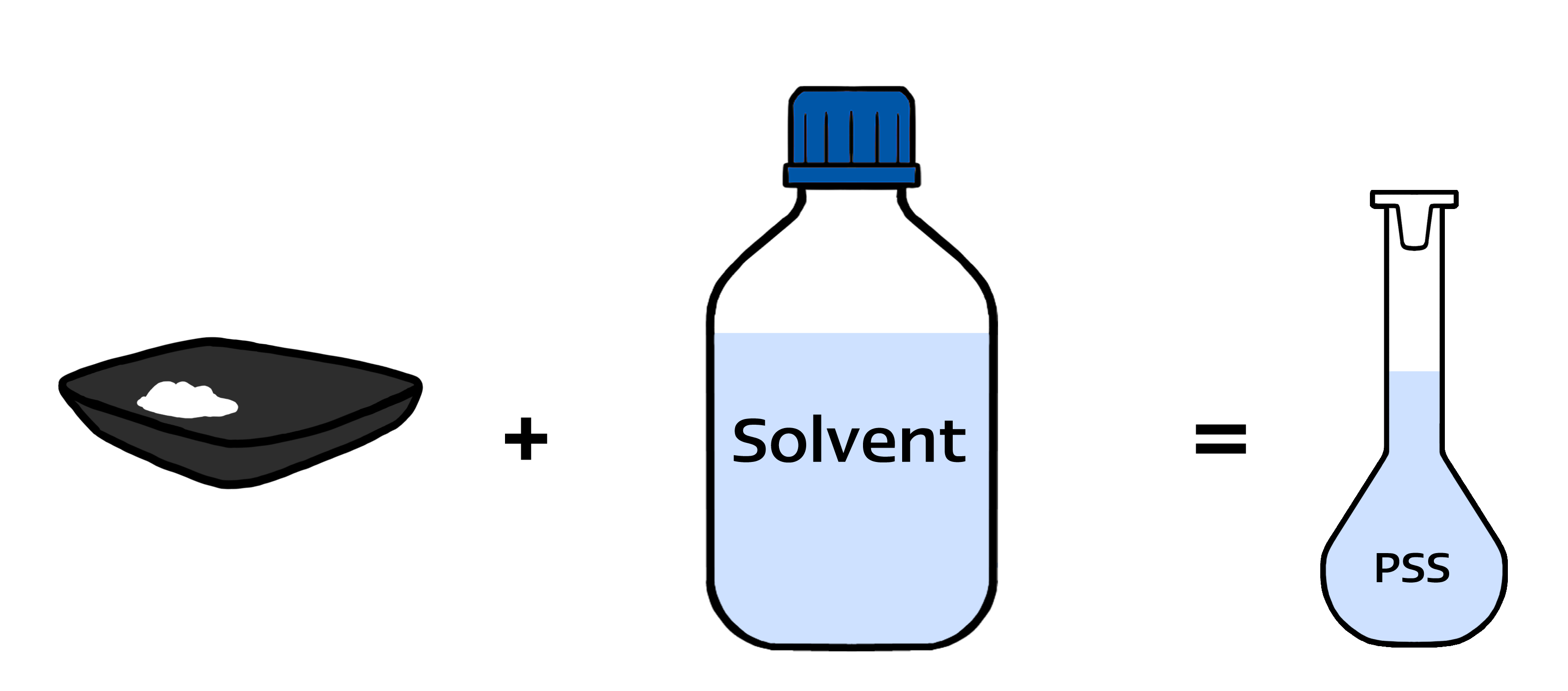
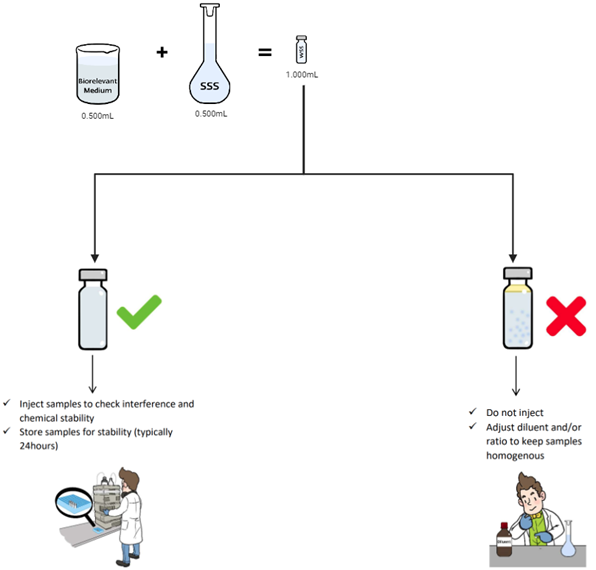
- Products
- View All Products
- FaSSIF/FeSSIF/FaSSGF
- FEDGAS
- FaSSIF-V2
- FeSSIF-V2
- Canine FaSSIF/FaSSGF
- FaSSCoF
- FeSSCoF
- Case Studies
- Learning CentreNew Posts!
- How To Buy
- Customer Reviews
- About Us
- Contact
0
0
ORDER ONLINE
OR EMAIL A PO

US SHIPMENTS
DELIVERED 2-3 DAYS

FREE SHIPPING
ORDERS OVER $999
Thank you!
You will shortly receive an email with a pdf of your quotation attached.
Get Quote
OUR CUSTOMERS INCLUDE :





Get Quote!
Your cart is empty.
Please add the items
you
want to buy,
then we can send a quote to you.

OK, got it


 Home
Home